Method for purifying urapidil with counter solvent recrystallization method
An anti-solvent recrystallization and urapidil technology, applied in the direction of organic chemistry, can solve the problems of low yield and long crystallization time, and achieve the effect of high yield, rapid precipitation and easy control of conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 5g of urapidil (purity 96.16%) into 15ml of dichloromethane, heat it at 40°C to dissolve it, and suction filter it while it is hot. White turbidity appeared, let it stand for 0.5h, vacuum-filtered under reduced pressure, and the obtained solid was vacuum-dried at room temperature to obtain 4.87g of urapidil, with a yield of 97.4% and a purity of 99.98%.
[0020] Product purity was detected by Shimadzu LC-10ATvp high performance liquid chromatography (SPD-10ATvp detector),
[0021] Detection method: National New Drug Standard [WS1-(X-116)-2003Z].
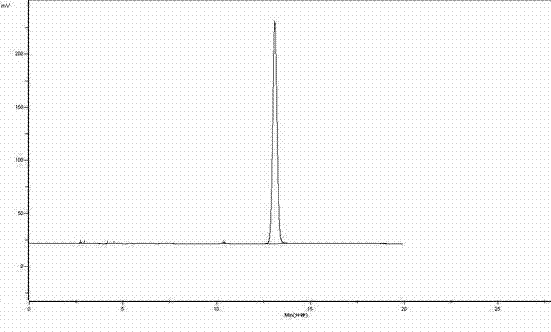
[0022] attached figure 1 The HPLC analysis result of urapidil standard substance is as follows:
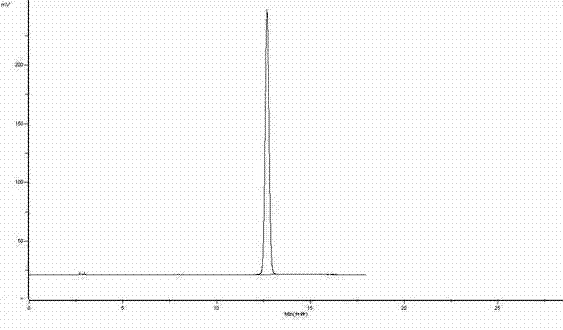
[0023] attached figure 2 The HPLC analysis result of urapidil before experimental example 1 anti-solvent recrystallization is as follows:
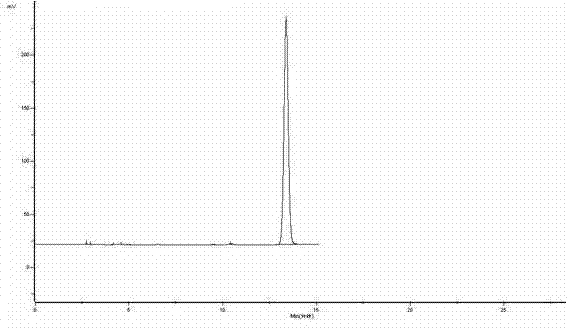
[0024] attached image 3 The HPLC analysis result of urapidil after experimental example 1 anti-solvent recrystallization is as follows:
[0025]
Embodiment 2
[0027] Add 5 g of urapidil (purity 96.16%) into 15 ml of DMSO, heat it at 45°C to dissolve it, and suction filter it while it is hot. Set aside for 0.5h, filter under reduced pressure, and dry the obtained solid under vacuum at room temperature to obtain 4.79g of urapidil, with a yield of 95.8% and a purity of 99.98%.
Embodiment 3
[0029] Add 5g of urapidil (purity 96.16%) into 15ml of THF, heat it at 45°C to dissolve it, and suction filter it while it is hot. White turbidity appeared rapidly, left standing for 0.5h, vacuum filtration under reduced pressure, and the resulting solid was vacuum-dried at room temperature to obtain 4.67g of urapidil with a yield of 93.4% and a purity of 99.93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com