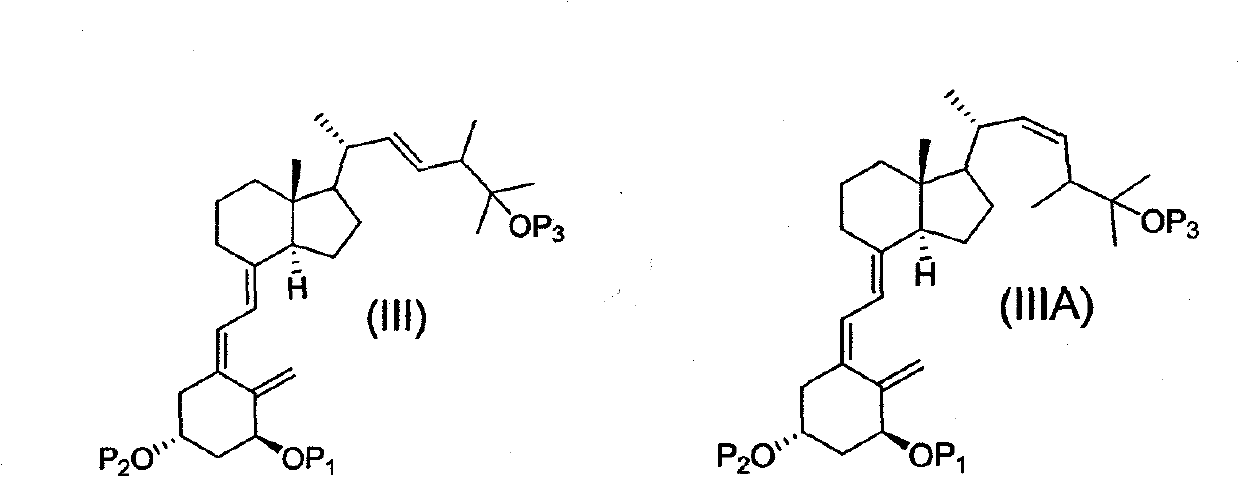
Method for synthesizing vitamin d analogues
A compound and general formula technology, applied in the field of synthesis of vitamin D2 analogues by photolysis and Wittig chemistry, can solve problems such as poor double bond formation selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1-cis alcohol intermediate 2
[0041]
[0042] The starting material trans alcohol 1 (6 g; 10.434 mmol) was placed in 9-acetylanthracene (0.597 g; 2.710 mmol), freshly distilled triethylamine (0.015 mL; 0.103 mmol) and 300 mL of toluene. in the flask. The resulting mixture was cooled to between about -1.7°C and 6°C and stirred under argon. The mixture was then exposed to light from a UV lamp inserted into a uranium filter glass tube. Aliquots of 100 μL were taken at 30 min, 45 min and 60 min intervals and analyzed for completeness by HPLC. The results shown in Table 1 below indicated that the reaction was complete within 30 minutes.
[0043] Table 1
[0044] point in time
[0045] Subsequently, the reaction mixture was transferred to a flask and evaporated under vacuum at 35°C. The residue was dissolved in dichloromethane (CH 2 Cl 2 ), loaded into a silica gel cartridge (cartridge), and washed with 0-15% diethyl ether (...
Embodiment 2
[0046] Embodiment 2: the preparation of aldehyde 3
[0047]
[0048] The cis-alcohol 2 was oxidized to the aldehyde with a sulfur trioxide pyridine complex as described in the literature (see Tojo and Fernandez, Oxidation of Alcohols to Aldehydes and Ketones, Springer (2006)). In 10 mL of freshly distilled triethylamine, 34 mL of CH 2 Cl 2 In the presence of 68 mL of dimethyl sulfoxide (DMSO), the cis-alcohol 2 (6.8 g, 11.82 mmol) was mixed with SO 3 • Py (9.4 g; 59.12 mmol) reacted. This reaction provided a quantitative yield of aldehyde 3.
Embodiment 3
[0049] Example 3: Cis Vitamin D 2 Preparation of Intermediate 5
[0050]
[0051] Phosphine oxide 4 (5.8 g, 13.92 mmol) in 75 mL dry tetrahydrofuran (THF) was cooled to about -78 °C in a dry ice / acetone bath under argon. After cooling for 10 minutes, butyllithium (11.14 mL, 27.84 mmol, 2.5M in hexanes) was slowly added via syringe. The resulting mixture was stirred at about -78°C for 45 minutes. Aldehyde 3 dissolved in 40 mL of anhydrous THF was then added to this mixture via syringe. The resulting mixture was stirred at -78°C for 45 minutes, then allowed to warm to about 0°C over 45 minutes to 1.5 hours. The reaction was then stopped, and 200 mL of ethyl acetate was added to the mixture, which was then washed with brine and water. The organic layer was dried over sodium sulfate, filtered and concentrated. The thick syrupy concentrate was dissolved in 200 mL of anhydrous THF and cooled to about -12°C in an ice-salt bath. To the cooled solution was added potassiu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com