Therapeutics for neurological disorders
A technology for therapeutic agents and motor neurons, applied in the field of therapeutic agents, can solve the problems of narrowing the high polarity of the therapeutic window
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
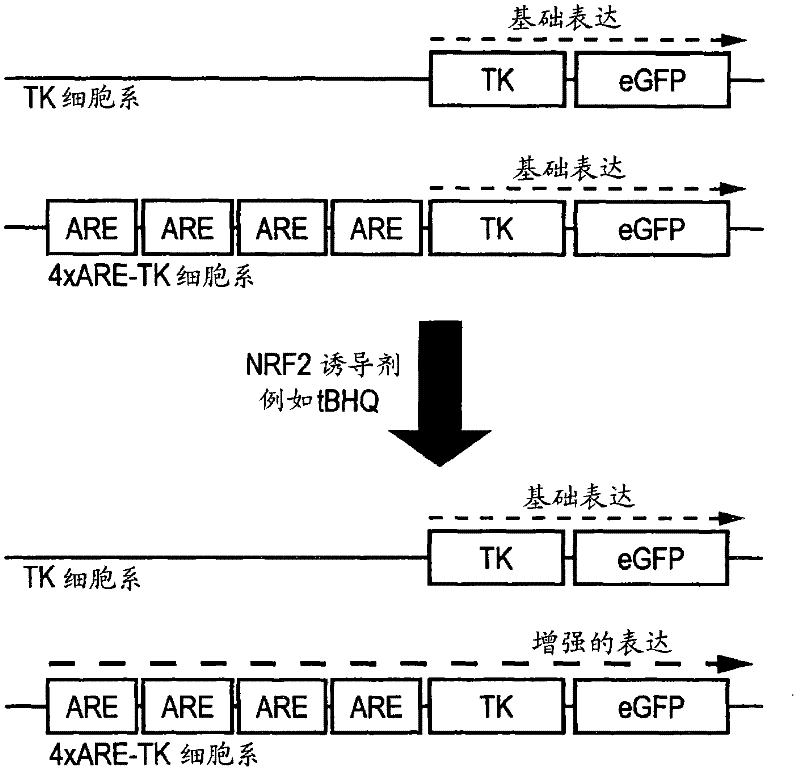
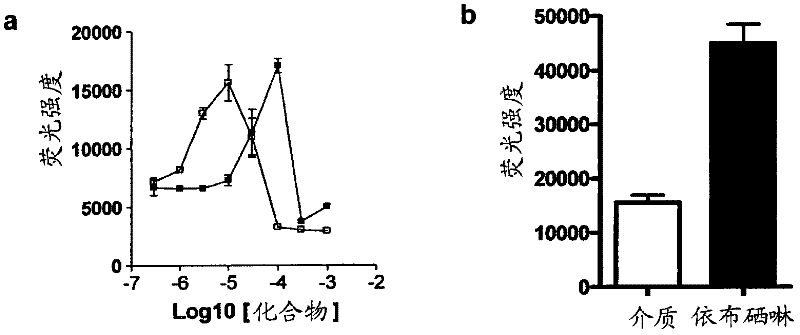
[0098] The 4×ARE-TK-GFP and TK-GFP reporter cell lines were tested for their response to a series of concentrations of the known ARE inducer tert-butyl hydroquinone (tBHQ) and the flavonoid EGCG. The compound was applied in triplicate to confluent cells in a serum-free culture in a 96-well plate for 24 hours. Detect the induction of GFP in a fluorescence plate reader. Both compounds induce GFP expression in a narrow window, with the peak value of EGCG at 100 μM and the peak value of tBHQ at 10 μM ( figure 2 a). At concentrations above this peak expression, both compounds showed signs of toxicity by direct observation (cell loss) or increased ethidium bromide dimer fluorescence. No increase in fluorescence was observed in the control TK-GFP cell line (not shown).
Embodiment 2
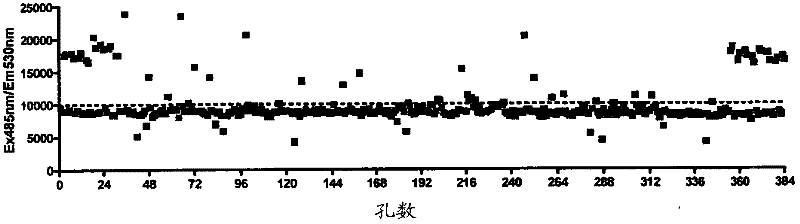
[0100] To screen the spectrum collection of 2000 molecules, the reporter assay was scaled down to a 384-well-plate format. In order to evaluate the suitability of the assay for library screening, Z'score calculation was performed by treating candidate wells with medium (0.1% DMSO) and 10 μM ebselen as a positive control (see calculation in method). We have shown that ebselen gives a strong concentration response curve in this determination. The calculated Z’ score is 0.51( figure 2 b), this is acceptable by the library screening. In addition, the signal-to-noise ratio (S / N) and the signal to background (S / B) ratio are acceptable, at 12.8 and 2.9, respectively. The library was then screened at a single concentration of 10 μM for each compound. The drug library dilution and plating were performed by the Q-BOT liquid handling system, and the reaction of the 4×ARE-TK-GFP and TK-GFP reporter cell lines to the compound was tested. image 3 An example set of ARE-TK-GFP cell line da...
Embodiment 3
[0102] To study the effect of Nrf2-ARE-induced sampling compounds on oxidative stress induced by serum deprivation in motor neurons and astrocytes. Since the activation of this pathway can vary with cell type, we continue to screen how well these sampled compounds protect motor nerve cell lines (NSC34 cells) and rat (C6) and human (1321N1) astrocytes It is free from oxidative stress induced by serum deprivation. The cell line was pretreated with a series of concentrations of sampling compounds for 24 hours to activate the NRF2-ARE pathway. The compound was then discarded, and the cells were subjected to serum deprivation for 6 hours to induce oxidative stress. Dichlorofluorescein (DCF) fluorescence was used to detect the degree of oxidative stress, and the degree of protection was shown in Table 3, as the percentage of DCF fluorescence of each of the three cell lines was reduced. If it is possible to fit the curve, you can also quote the semi-maximum effect (IC 50 ) The requi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com