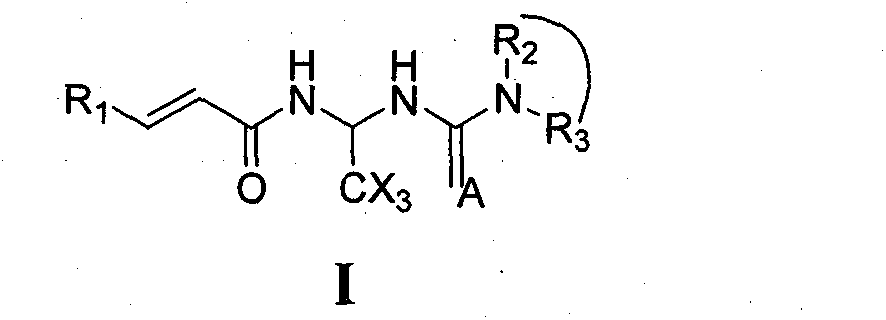
Carbamide compound and its medicinal usage
A technology of compounds and medicinal salts, applied in organic chemistry, drug combination, cardiovascular system diseases, etc., can solve problems such as low selectivity and targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 (2E)-3-Phenyl-N-[1-(8-quinolylamino)oxocarboxamido-2,2,2-trichloroethyl]-2-acrylamide
[0057]
[0058] Dissolve 6.50 g of cinnamic amide and 8.01 g of chloral hydrate in 300 ml of toluene, and reflux at 110°C for 8 hours to prepare intermediate (2E)-3-phenyl-N-[1-hydroxyl-2,2, 2-Trichloroethyl]-2-acrylamide, dissolve 4.00g of (2E)-3-phenyl-N-[1-hydroxyl-2,2,2-trichloroethyl]-2-acrylamide in In 40ml of anhydrous THF, add 4.9ml of SOCl dropwise at room temperature 2 , After dropping, heated to reflux at 60°C for 3 hours. Evaporate the solvent to dryness, dissolve in 20ml of anhydrous ether, drop into 20ml of concentrated ammonia water at 0°C, and stir for 30min. The layers were separated and evaporated to dryness to obtain 2.93 g of the intermediate (2E)-3-phenyl-N-[1-amino-2,2,2-trichloroethyl]-2-acrylamide. Dissolve 0.58g of 8-aminoquinoline in 8ml of anhydrous THF, add 1ml of DIEA, cool to -10°C, drop in 0.56ml of trichloroethyl chloroformate, and rea...
Embodiment 2
[0059] Example 2 (2E)-3-Phenyl-N-[1-(4-methylphenylamino)oxocarboxamido-2,2,2-trichloroethyl]-2-acrylamide
[0060]
[0061] Using the method of Example 1, the 8-aminoquinoline was changed to p-methylaniline to obtain 0.17 g of white solid. 1 H-NMR (400MHz, DMSO-d 6 )δ2.22(s, 3H); δ6.62-6.67(t, 1H); δ6.91-6.95(d, 1H); δ7.04-7.06(d, 2H); δ7.30-7.33(d , 2H); δ7.40-7.61 (m, 7H); δ9.18-9.20 (d, 1H); δ9.59 (s, 1H). MS (TOF) 448.0 (M+).
Embodiment 3
[0062] Example 3 Compound protection cardiomyocyte activity experiment
[0063] Cardiomyocyte primary culture
[0064] Cardiomyocytes were isolated and cultured according to the method of differential adhesion separation (Kreider, A.Messing, H.Doan, S.U.Kim, R.P.Lisak and D.E.Pleasure, Enrichment of Schwann cell cultures from neonatal rat sciaticnerve by differential adhesion, Brain Res 2 (1981), pp.433444.), took newborn Wistar suckling mice within 24 hours, disinfected the skin of the chest and abdomen with iodine alcohol, used scissors to open the chest slightly to the left of the midline under the xiphoid process, obliquely opened the chest, took out the heart and placed it on ice. Pre-cooled PBS; use 0.01M PBS to gently pipette the heart to remove blood cells and other tissues, and cut the heart into 0.5mm 3 For fragments of different sizes, wash repeatedly 2-3 times with 0.01M PBS; place the fragments in an Erlenmeyer flask, add 4ml 0.125% trypsin, 1ml 0.1% collagen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com