Dry powder administration device
A drug delivery device and dry powder technology, applied in the direction of inhalers, etc., can solve the problems of moisture in the dry powder of the drug, affecting the accuracy and use effect of the drug dosage, and the difficulty in the measurement of the storage type drug, so as to ensure the dosage of the drug and reduce the moisture. and agglomeration chances and possible effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0070] The present invention will be further described below in conjunction with the accompanying drawings.
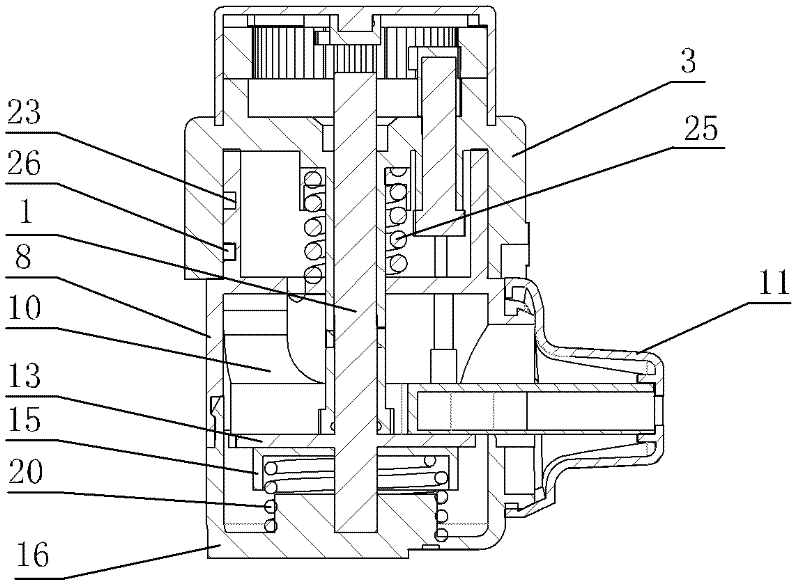
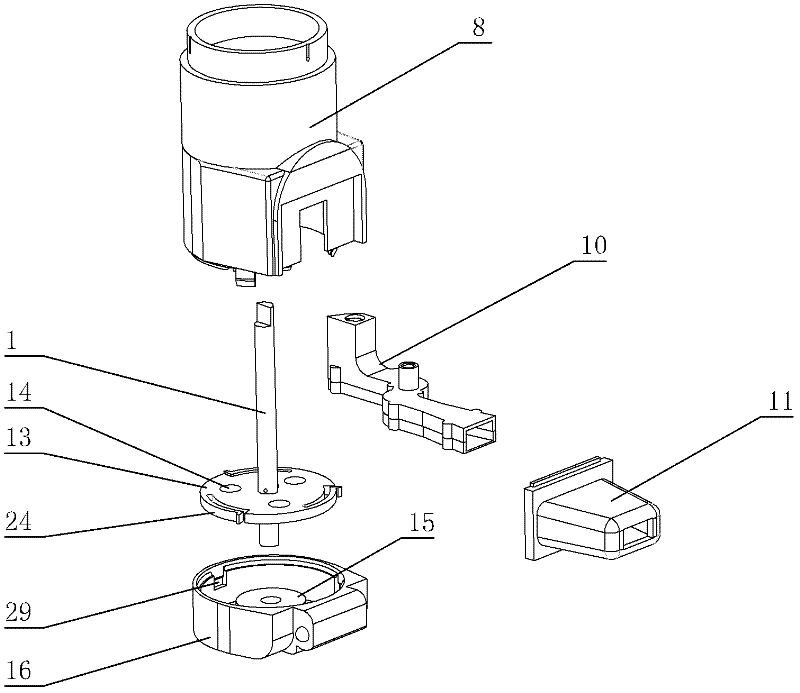
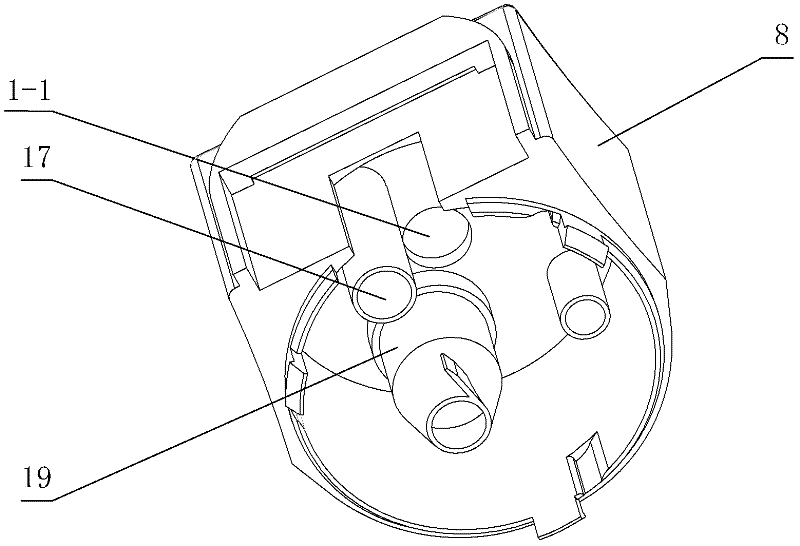
[0071] figure 1 and figure 2 Among them, the dry powder drug delivery device is provided with a housing composed of an upper main body part 8 and a lower main body part 16; on the outer periphery of the upper part of the housing, an annular manual rotating part 3 is set; on the outer surface of the upper part of the housing, a The rotating slideway 23 is provided with a slide block 26 on the inner surface of the ring-shaped manual rotating part; between the ring-shaped manual rotating part and the upper part of the housing, a first elastic member 25 is arranged; at one end of the rotating slideway, An air inlet is provided; inside the housing, a flow channel part 10 is provided; on the outside of the housing, a suction nozzle 11 is provided, the head end of the flow channel part is connected to the gas path of the air inlet, and the end of the flow channel part is co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com