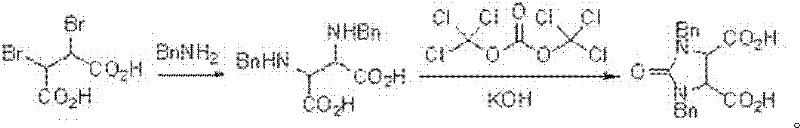Method for synthesizing 1,3-dibenzyl imidazoline-2-keto-cis-4,5-dicarboxylic acid
A technology of dibenzyl imidazoline and dicarboxylic acid, applied in the direction of organic chemistry, can solve the problems of harsh labor protection conditions, restrictions on phosgene transportation and storage, cumbersome recovery of excess benzylamine, etc., to improve the production environment, impurity Less, less expensive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1. Synthesis of dibenzylurea
[0036] In a 250ml four-neck flask, add 20g (333.00mmol) of urea, 85.64g (799.20mmol) of benzylamine, 2.22g (16.65mmol) of aluminum chloride and 100ml of DMF, and heat up to 100°C after argon replacement, and react for 30 minutes . The reaction solution was cooled to 20° C., then poured into a beaker containing 50 g of crushed ice, and stirred for 30 minutes. Suction filtration, washing with 2*20ml of water, and vacuum drying yielded 76.18g of dibenzylurea (molar yield: 95.2%), white solid, melting point: 167.2-168.3°C.
[0037] 1 H-NMR (DMSO-d 6 , 400MHz): δ 4.46 (d, 2H), 4.75 (dd, 4H), 7.29-7.40 (m, 10H). 13 C-NMR (DMSO-d 6 ): δ158.8, 139.3, 128.8, 127.4, 44.5.
[0038] 2. Synthesis of target product 1,3-dibenzyl imidazolin-2-one-cis-4,5-dicarboxylic acid
[0039]In a 500ml four-necked flask, 20g (83.23mmol) of dibenzylurea, 22.96g (83.23mmol) of cis-2,3-dibromosuccinic acid and 160g of acetic acid were added. After argon replacem...
Embodiment 2
[0049] The only difference between this example and Example 1 is that "22.96g (83.23mmol) cis-2,3-dibromosuccinic acid" is replaced by "15.56g (83.23mmol) cis-2,3-dibromosuccinic acid" Chlorobutacic acid ", all the other contents are identical with those described in Example 1.
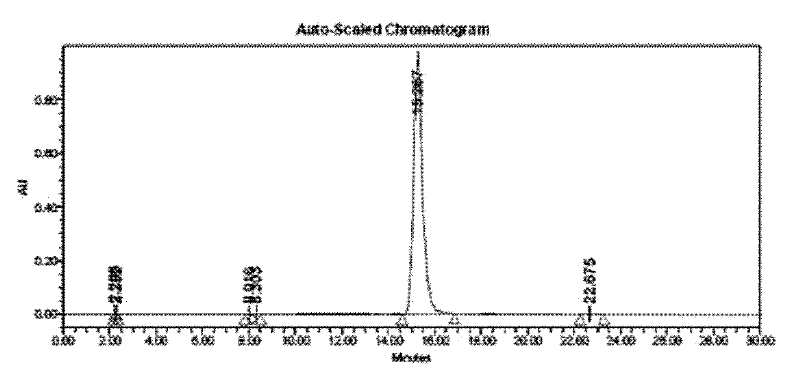
[0050] According to the HPLC purity analysis conditions described in Example 1, the analysis shows that the HPLC purity of the target product obtained by the method of this example is 99.73%.
Embodiment 3
[0052] The difference between this embodiment and Example 1 is only: "2.22g (16.65mmol) aluminum chloride" is replaced by "2.36g (16.65mmol) boron trifluoride ether", and the rest of the content is the same as in Example 1 Same as described.
[0053] According to the HPLC purity analysis conditions described in Example 1, the analysis shows that the HPLC purity of the target product obtained by the method of this example is 99.62%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com