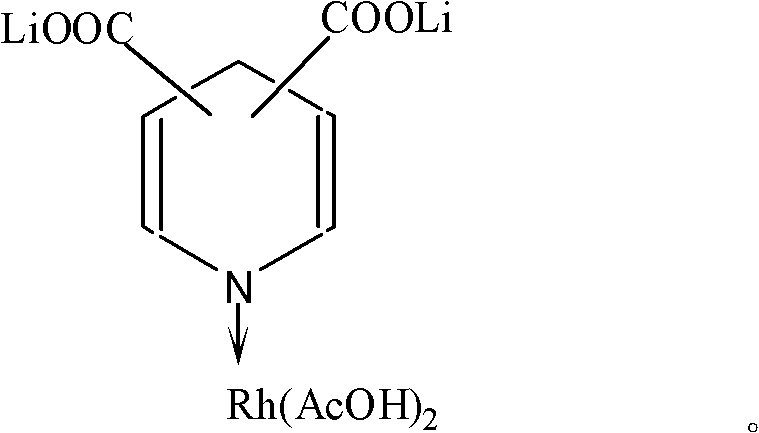
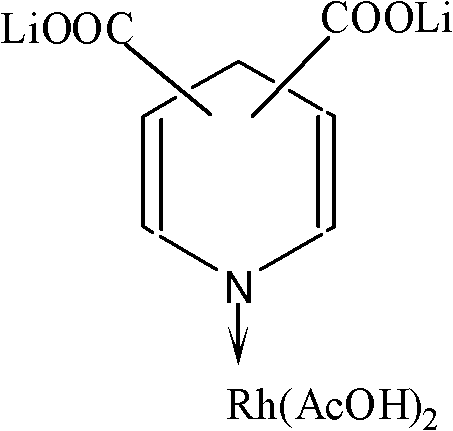
Lithium pyridine carboxylate-rhodium acetate complex catalyst for synthesizing acetic acid and acetic anhydride through carbonylation, and preparation method and application thereof
A technology of lithium pyridine carboxylate and catalyst, which is applied in the direction of organic compound/hydride/coordination complex catalyst, catalyst activation/preparation, carboxylic anhydride preparation, etc. It can solve the problems of catalyst instability and achieve excellent catalytic activity and reaction The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Dissolve 1 molar part of pyridine-2,3 lithium dicarboxylate in 100 molar parts of acetic acid, add 1 molar part of rhodium acetate under stirring, continue stirring for about 20 minutes, then add an excess amount of diethyl ether relative to the volume of the reaction product Precipitate and filter to obtain pyridine-2,3 dicarboxylate lithium-rhodium acetate complex catalyst solid.
[0021] Using the same method as above, replace lithium pyridine-2,3 dicarboxylate with lithium pyridine-2,6 dicarboxylate or lithium pyridine-3,5 lithium dicarboxylate to obtain pyridine-2,6 lithium dicarboxylate- Rhodium acetate complex catalyst or pyridine-3,5 lithium dicarboxylate-rhodium acetate complex catalyst.
Embodiment 2
[0023] Dissolve 1 molar part of pyridine-2,6 dicarboxylate lithium in 200 molar parts of acetic acid, add 1 molar part of rhodium acetate under stirring, continue stirring for about 20 minutes, then add an excess amount of diethyl ether relative to the volume of the reaction product Precipitate and filter to obtain pyridine-2,6 dicarboxylate lithium-rhodium acetate complex catalyst solid.
[0024] Using the same method as above, replace lithium pyridine-2,6 dicarboxylate with lithium pyridine-2,3 lithium dicarboxylate or lithium pyridine-3,5 dicarboxylate to obtain pyridine-2,3 lithium dicarboxylate- Rhodium acetate complex catalyst or pyridine-3,5 lithium dicarboxylate-rhodium acetate complex catalyst.
Embodiment 3
[0026] In a 250ml autoclave, add pyridine-2 prepared in Example 1, 0.30 g of lithium dicarboxylate-rhodium acetate complex catalyst, 0.8 mol of methanol, 0.19 mol of methyl iodide, 1.12 mol of acetic acid, and 2.1 g of lithium iodide; Carbon monoxide was introduced, the temperature was raised to 150° C., the stirring speed was 500 rpm, the reaction pressure was controlled at 3.0 MPa, and the reaction time was 13 minutes to obtain acetic acid. The conversion rate of methanol is 96%, and the space-time yield of acetic acid is 20.1molAcOH / L.h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com