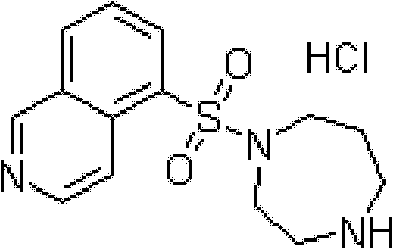
Fasudil hydrochloride injection composition and preparation method thereof
A technology for fasudil hydrochloride and a composition, which is applied in the directions of drug delivery, pharmaceutical formulations, and medical preparations containing active ingredients, can solve problems such as poor stability, quality defects, and difficulty in reaching the expiration date.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0025] Experimental example one: measure the millimolar osmotic pressure experiment of pharmaceutical composition of the present invention (prepared in embodiment one)
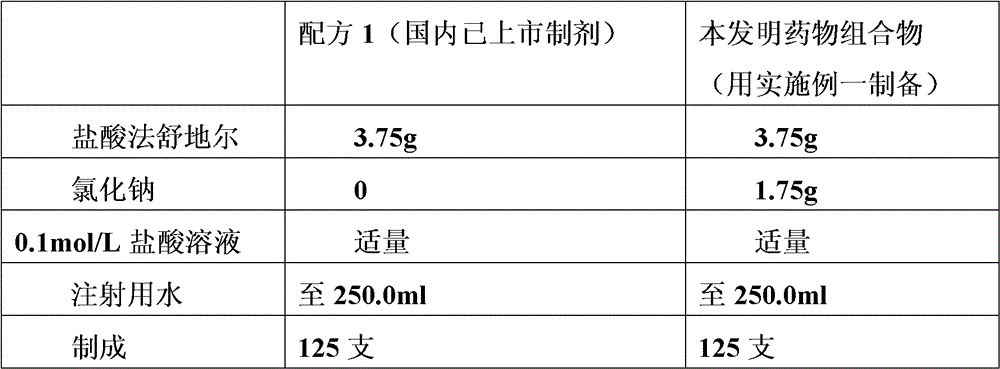
[0026] The formula consists of:
[0027]
[0028] Note: * is the pharmaceutical composition of the present invention prepared in Example 1.
[0029] Each formulation uses the same preparation method: add fasudil hydrochloride and solid excipients to an appropriate amount of water for injection, shake to dissolve, use 0.1mol / L hydrochloric acid solution to adjust the pH of the drug solution to 3.6-3.9, and accurately transfer the drug solution into 250ml In the measuring bottle, add water for injection to the mark, shake well, transfer the solution into a beaker, use a filter with a pore size of 0.22 μm to filter and fill, the volume of each bottle is 1.80 ~ 2.20ml (the ampoule has been cleaned and sterilized), melt and seal, Autoclaving at 121°C for 15 minutes, and light inspection. Check the prope...
experiment example 2
[0032] Experimental example 2: freeze-thaw test
[0033] The formula consists of:
[0034]
[0035] Each formulation uses the same preparation method: add fasudil hydrochloride and solid excipients to an appropriate amount of water for injection, shake to dissolve, use 0.1mol / L hydrochloric acid solution to adjust the pH of the drug solution to 3.6-3.9, and accurately transfer the drug solution into 250ml In the measuring bottle, add water for injection to the mark, shake well, transfer the solution into a beaker, use a filter with a pore size of 0.22 μm to filter and fill, the volume of each bottle is 1.80 ~ 2.20ml (the ampoule has been cleaned and sterilized), melt and seal, Samples were autoclaved at 121°C for 15 minutes and inspected by light.
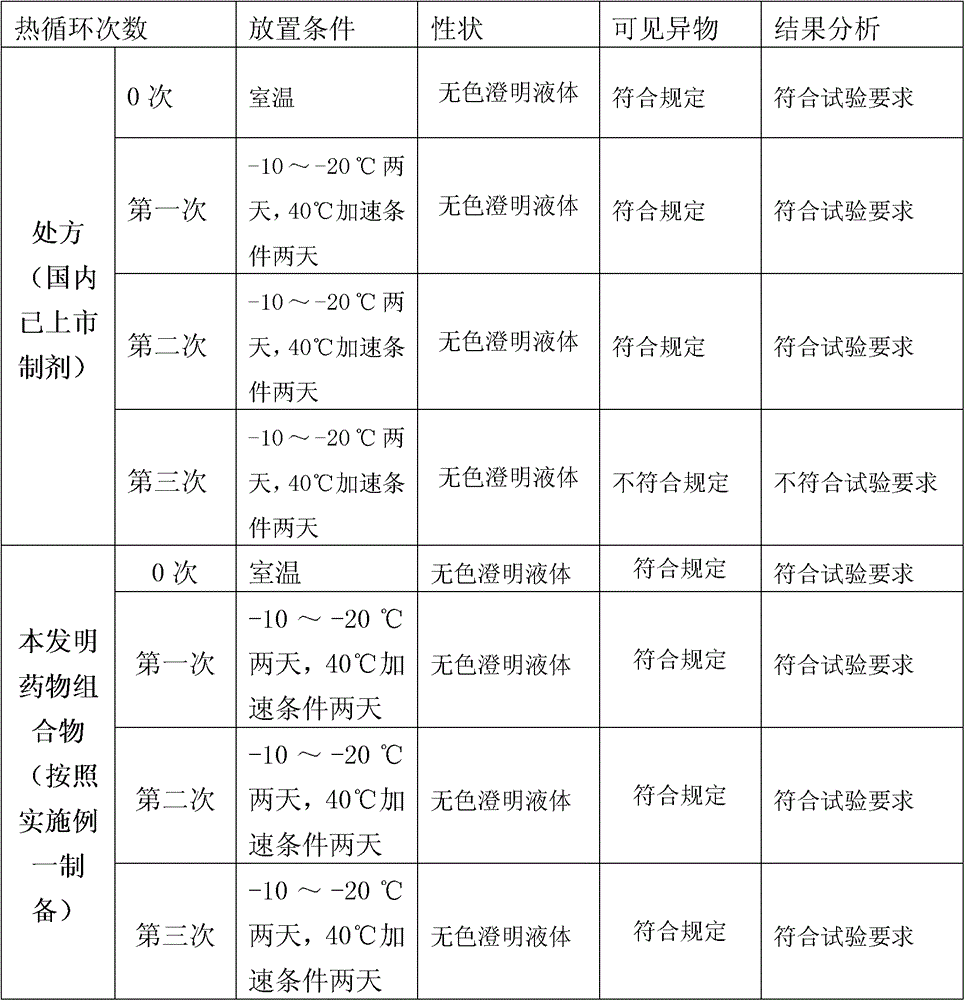
[0036] Place the samples according to the freeze-thaw test conditions, the specific method is as follows:
[0037] The freeze-thaw test should include three cycles, each cycle should be under the condition of -10~-20°C for 2 d...
experiment example 3
[0042] The experiment was done in 2 groups, and its samples were respectively the pharmaceutical composition of the present invention (same as experimental example 2) and domestic marketed preparations (same as experimental example 2).
[0043] Each group takes 3 rabbits that are healthy and qualified and have no damage to the ear margins, administer the drug according to body weight, and inject 5ml / kg of this product into the left ear margin vein with an aseptic method [During the test, take 5 bottles of this product, add Dilute and dissolve N.S to 50ml to 0.6mg / ml for testing], inject the same volume of N.S on the right side in the same way, and the injection speed is about 2ml / min. 1 time per day for a total of 3 days. Every day before administration and 48-96 hours after the last administration and on the 14th day after the end of the observation period, observe whether there is congestion, erythema, and edema in the tissues and blood vessels below the proximal end of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com