New crystal form of lansoprazole and preparation method and application thereof
A technology of lansoprazole and its crystal form, which is applied in the field of new lansoprazole crystal form and its preparation, can solve the problems of complex preparation process and time-consuming, and achieve simple operation, less solvent amount and good reproducibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The preparation method of Lansoprazole M crystal form, comprises the following steps:
[0038] 1) Lansoprazole is dissolved in methanol: in the mixed solvent of water=5:3 (volume ratio), filter;
[0039]2) Cool the filtrate to -20~-15°C to precipitate crystals;
[0040] 3) The crystals are filtered and dried to obtain the M crystal form of lansoprazole.
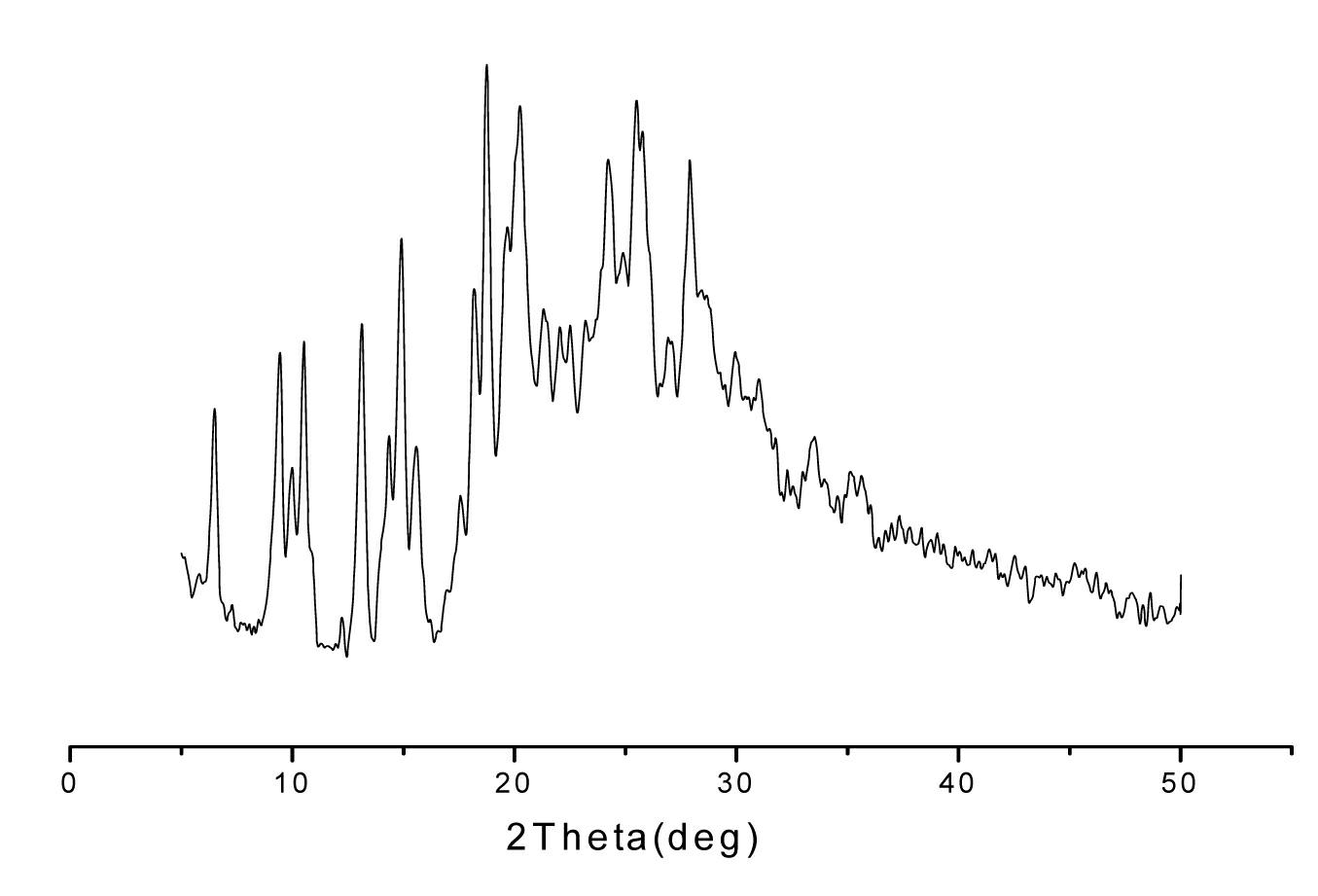
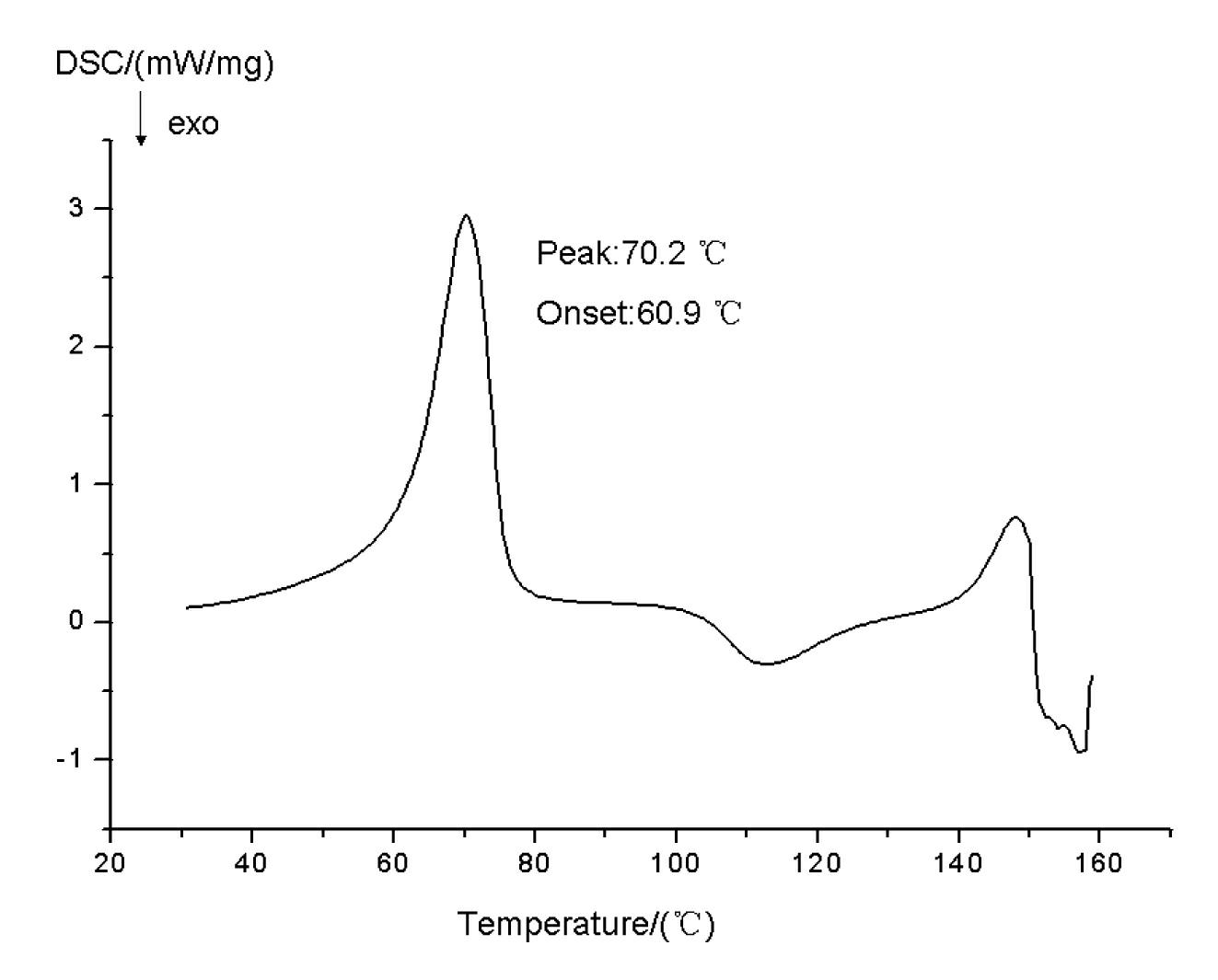
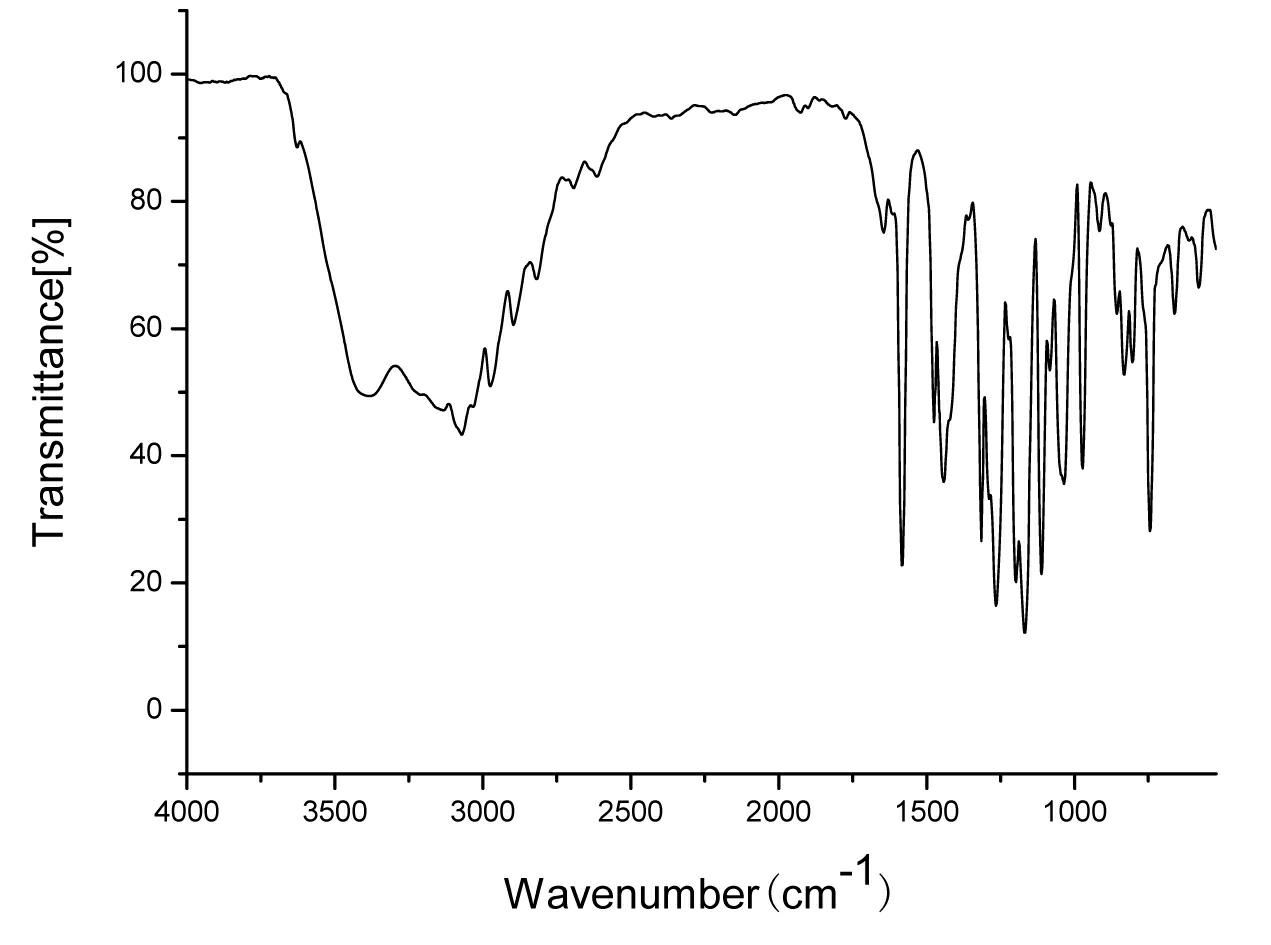
[0041] Lansoprazole N crystal form, its X-ray powder diffraction at diffraction angle 2 θ For: 5.438, 7.062, 8.230, 9.216, 11.022, 11.789, 12.610, 13.541, 20.603, 21.862, 26.242 have characteristic peaks; 1 H-NMR (CDCl 3 ) data are: 8.34 (d, 1H), 7.65 (br., 2H), 7.30 (d, 1H), 6.67 (d, 1H), 4.74 (q, 2H), 4.40 (d, 1H), 4.32 (d , 1H), 2.21 (s, 3H); Its IR (KBr, cm -1 ) data are: 3628, 3382, 3133, 3071, 2974, 2896, 2818, 1645, 1582, 1475, 1441, 1315, 1265, 1198, 1168, 1111, 1036, 972, 916, 857, 744, 661.
[0042] The preparation method of Lansoprazole N crystal form, comprises the following steps:
[0043] 1) Lansop...
Embodiment 1
[0049] 1) 50mg lansoprazole was dissolved in 0.1mL methanol solution;
[0050] 2) Add 0.06 mL of water dropwise to the resulting solution, and filter;
[0051] 3) Cool down the clear solution to -15°C to make it fully crystallized, filter and dry.
[0052] Obtained 43.1 mg of off-white crystalline powder with a yield of 86.2%.
Embodiment 2
[0054] 1) 100mg lansoprazole was dissolved in 0.5mL methanol solution;
[0055] 2) Add 0.3 mL of water dropwise to the resulting solution, and filter;
[0056] 3) Cool down the clear solution to -15°C to make it fully crystallized, filter and dry.
[0057] 87.5 mg of off-white crystalline powder was obtained, with a yield of 87.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com