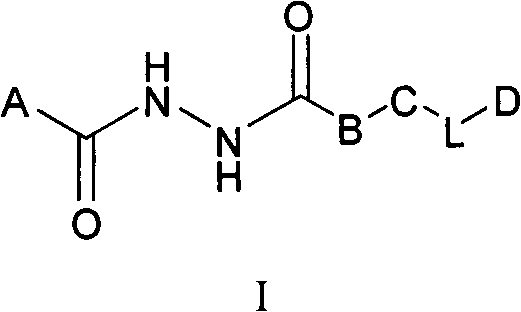
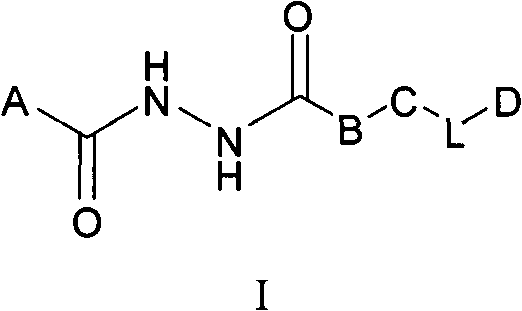
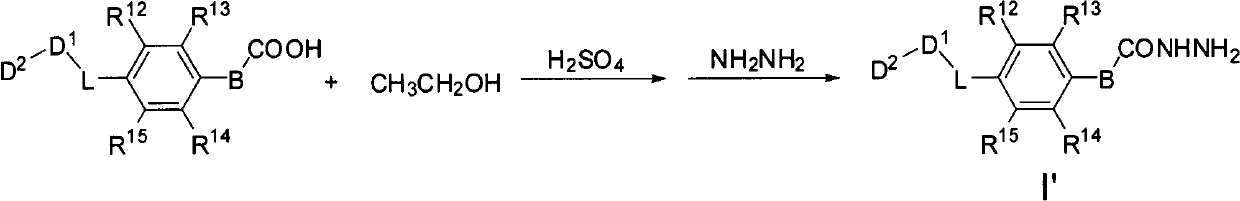
Aromatic dihydrazide type PLK1 (Polo-like Kinase 1) inhibitor and applications thereof
A kind of technology of aryl, methylsulfonyl piperazinyl methyl, applied in the field of medicinal chemistry, can solve the problem of not many types of structures and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0197] 3,4,5-Trimethoxybenzohydrazide (I'-1)
[0198] Add 15.15g (0.1mol) of 3,4,5-trimethoxybenzoic acid and 30ml of methanol into a 100ml three-necked flask, stir at room temperature, and slowly add excess SOCl dropwise 2 (20ml), then heated to 70 ° C, reflux for 4h, and then evaporated under reduced pressure to remove unreacted SOCl 2 And methanol, after cooling, a white solid precipitates out, add saturated Na 2 500ml of CO3 solution, filtered by suction and dried by infrared, directly proceeded to the next step without purification.
[0199] Dissolve 8.0 g of the solid obtained in the previous step in 15 ml of methanol, slowly add 15 ml of hydrazine hydrate, and a white solid precipitates out during the dropwise addition. After the addition, 5ml of methanol was added to dilute the reaction solution. After stirring at 100°C for 1 h, TLC detected that new substances were formed, and the raw material ester basically disappeared. After cooling to room temperature, filter ...
Embodiment 2
[0201] p-Tolylhydrazide (I'-2)
[0202] The preparation method was similar to (I'-1), and 6.0 g of the sample was obtained, with a yield of 90%, mp.116-118°C (literature value 116-118°C).
Embodiment 3
[0204] 4-hydrazinobenzoic acid (I'-3)
[0205] The preparation method was similar to (I'-1), and 1.68 g of the sample was obtained, with a yield of 93%, and mp.230-232°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com