Preparation method of hydroxyl pyridine compound
A hydroxypyrimidine and compound technology, applied in the direction of organic chemistry, can solve the problems of low solubility, slow reaction speed, low yield, etc., and achieve the effects of increasing solubility, increasing yield and content, and accelerating production speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
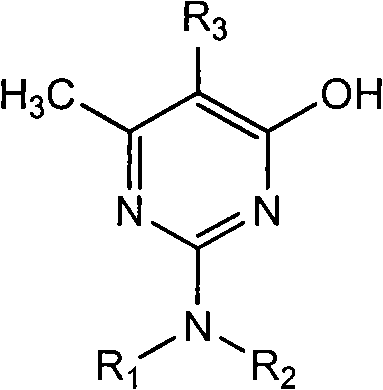
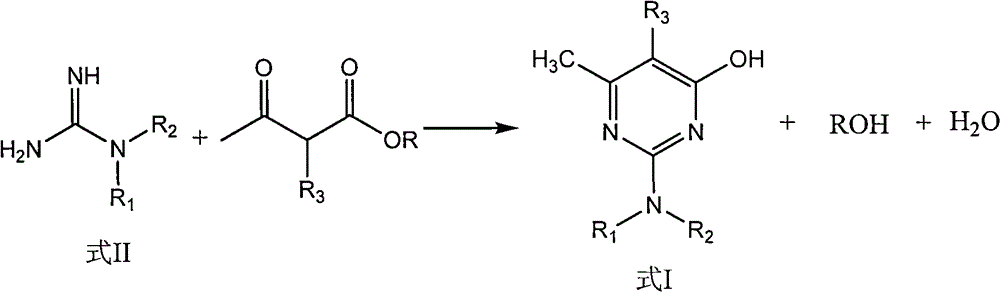
[0025] Example 1: Synthesis of 2,2-dimethylamino-5,6-dimethyl-4-hydroxyl-pyrimidine
[0026] In the 500ml three-necked reaction flask that stirring, thermometer, reflux condenser are housed, add 200ml toluene, 30ml methyl alcohol, start stirring, add 15.0g (0.05mol) metguanidine sulfate and 4.8g (0.115mol) solid sodium hydroxide, After keeping stirring at 20-40°C for 2 hours, start to add 16.4g (0.12mol) methyl a-methyl acetoacetate dropwise, raise the temperature to reflux after dropping, separate the water and methanol, continue to raise the temperature and react until the boiling point of the solvent, and the reaction ends. Cool down to below 100°C, add 30ml of water and 1g of concentrated sulfuric acid, stir for 15 minutes, adjust the pH of the reaction solution to 7, separate the water layer at rest, remove the solvent from the oil layer under reduced pressure to obtain 16.2g of off-white solid product, content: 98.0%, yield : 95.1%.
Embodiment 2
[0027] Example 2: Synthesis of 2,2-dimethylamino-5,6-dimethyl-4-hydroxyl-pyrimidine
[0028] In the 500ml three-necked reaction flask that stirring, thermometer, reflux condenser are housed, add 200ml toluene, 20ml ethanol, start stirring, add 15.0g (0.05mol) metguanidine sulfate and 4.8g (0.115mol) solid sodium hydroxide, Keep stirring at 20-40°C for 2 hours, start to add 18.1g (0.12mol) ethyl a-methyl acetoacetate dropwise, raise the temperature to reflux after dropping, separate the water and ethanol, continue to raise the temperature to react to the boiling point of the solvent, and the reaction ends. Cool down to below 100°C, add 30ml of water and 1.3g of concentrated sulfuric acid, stir for 15 minutes, adjust the pH of the reaction solution to 7, separate the water layer at rest, remove the solvent from the oil layer under reduced pressure to obtain 16.3g of off-white solid product, content: 97.5%, yield Rate: 95.2%.
Embodiment 3
[0029] Example 3: Synthesis of 2,2-dimethylamino-5,6-dimethyl-4-hydroxyl-pyrimidine
[0030] In a 500ml three-neck reaction flask equipped with stirring, thermometer and reflux condenser, add 200ml xylene and 30ml methanol, start stirring, add 15.0g (0.05mol) metguanidine sulfate and 4.8g (0.115mol) solid sodium hydroxide , keep stirring at 20-40°C for 2 hours, start dropwise adding 16.4g (0.12mol) methyl a-methyl acetoacetate, raise the temperature to reflux after dropping, separate the water and methanol, continue to heat up to the boiling point of the solvent, and the reaction is over , cooled to below 100°C, added 30ml of water and 1g of concentrated sulfuric acid, stirred for 15 minutes, adjusted the pH=7 of the reaction solution, separated the water layer at rest, and decompressed the oil layer to remove the solvent to obtain 16.4g of off-white solid product, content: 98.2%. Rate: 96.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com