Application of astragaloside IV in preparing liver cancer resisting medicines
A technology of astragaloside and anti-liver cancer, which is applied in drug combination, antineoplastic drugs, and pharmaceutical formulations, and can solve problems such as control, quality and stability, and no reports and publications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
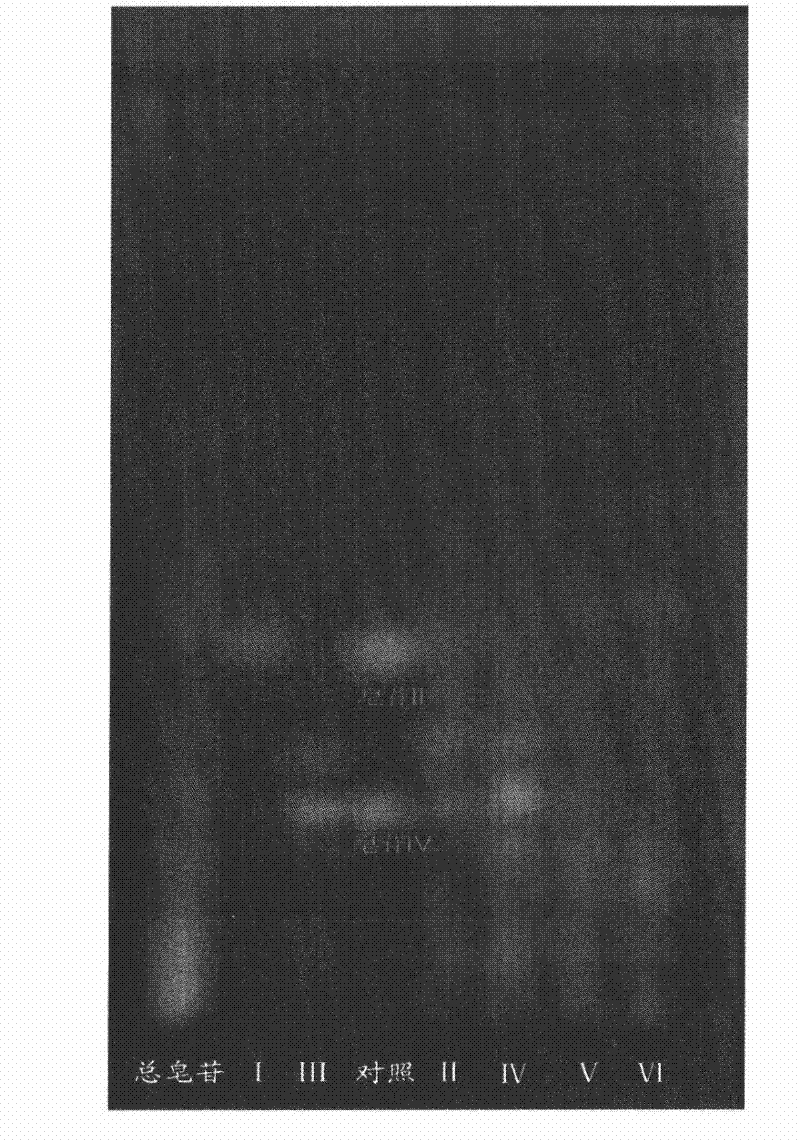
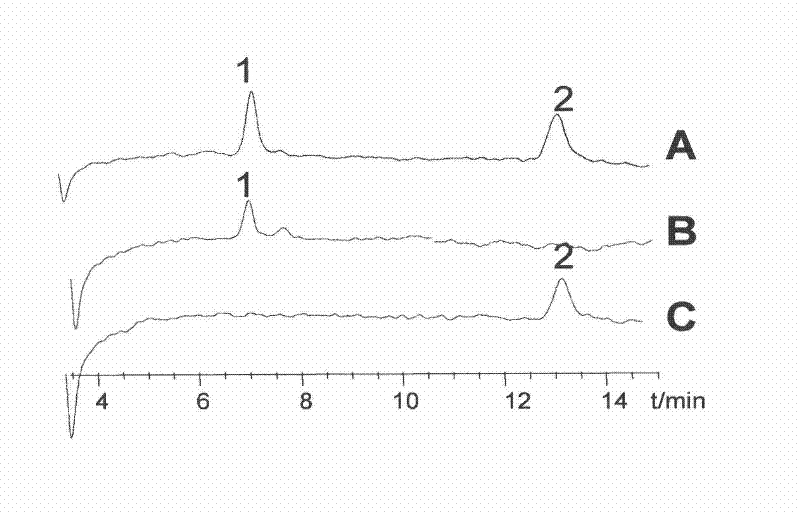
[0054]Example 1 Preparation of Astragaloside II and IV
[0055] 1. Experimental materials
[0056] 1.1 Astragalus membranaceus test drug (origin in Gansu, purchased from SDIC Pharmaceutical Anhui Co., Ltd., batch number: 080623; identified as Astragalus membranaceus by Professor Liu Shoujin from the Teaching and Research Office of Medicinal Botany, Anhui College of Traditional Chinese Medicine); Product Testing Institute, batch number: 0781-200210); astragaloside Ⅱ standard product (provided by Shanghai Traditional Chinese Medicine Standardization Research Center, batch number: 07-1015); silica gel high-efficiency G plate (Qingdao Ocean Chemical Factory); silica gel for column chromatography (Qingdao Ocean Chemical Industry Group); D101 resin (Tianjin Youchang Industry and Trade Co., Ltd.); ethanol, n-butanol, ethyl acetate, and methanol were analytically pure, acetonitrile was chromatographically pure, and water was double-distilled water.
[0057] 1.2 Instruments
[0058] ...
Embodiment 2
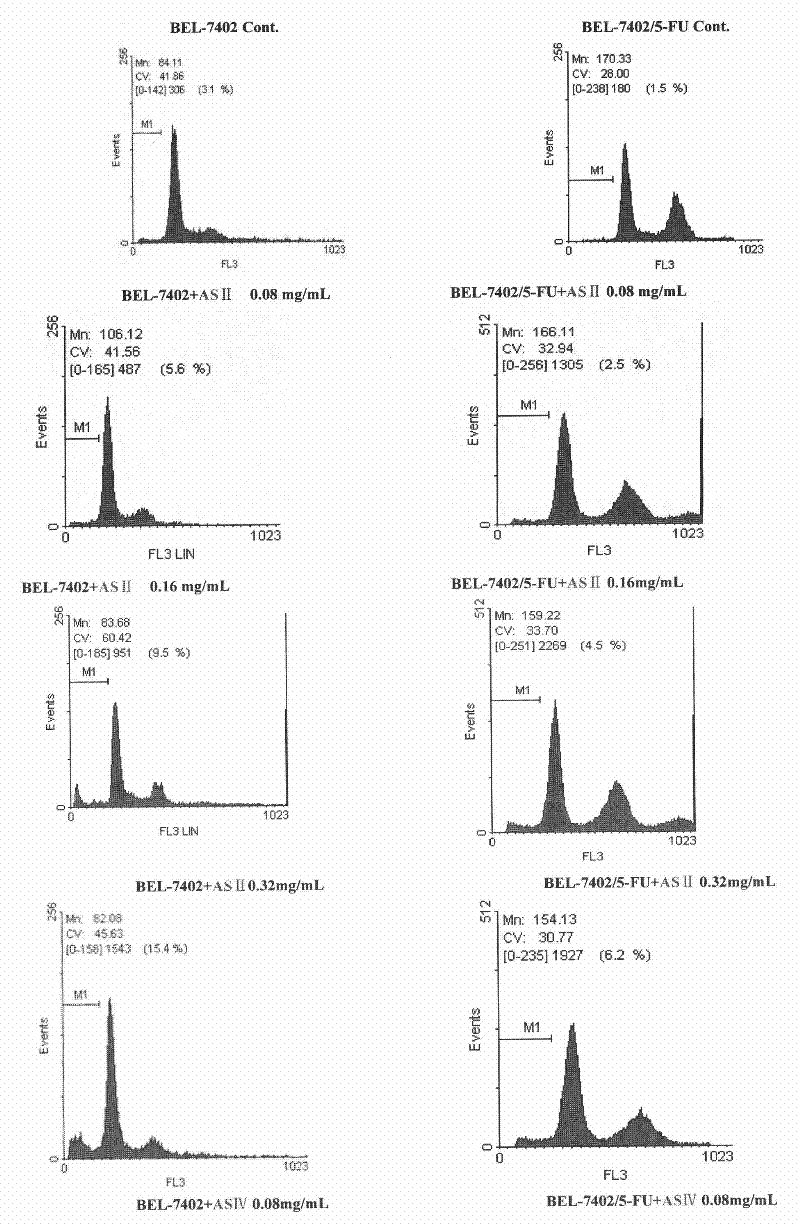
[0103] Example 2 Study on Antitumor Effects of Astragalus Saponins Ⅱ and Ⅳ
[0104] 1. Experimental materials
[0105] 1.1. Cell lines Human liver cancer cell line BEL-7402 and liver cancer drug-resistant cell line BEL-7402 / 5-FU were purchased from Nanjing KGI Biotechnology Development Co., Ltd.
[0106] 1.2 Drugs and reagents
[0107] Astragaloside Ⅱ (AS Ⅱ), Astragaloside Ⅳ (AS Ⅳ) (Laboratory self-made)
[0108] D-verapamil (R(+)-VERAPAMIL HCL, VRP) was purchased from IL Company, USA, with a purity of >99%
[0109] 5-FU Tianjin Jinyao Amino Acid Co., Ltd., batch number 0810211
[0110] MMC Zhejiang Hisun Pharmaceutical Co., Ltd., batch number 0810211
[0111] ADR Zhejiang Hisun Pharmaceutical Co., Ltd., batch number 0810211
[0112] DMEM and fetal bovine serum American Gibco products
[0113] MTT American Sigma products
[0114] DMSO American Sigma products
[0115] Rho123 Product of Sigma, USA
[0116] Trypsin BIOSHARP products
[0117] PBS Boster Biological Co., Ltd...
Embodiment 3
[0249] Tablet: Astragaloside IV 10mg
[0250] Lactose 187mg
[0251] Corn starch 50mg
[0253] Preparation method: mix the active ingredient, lactose and starch, moisten it evenly with water, sieve the wetted mixture and dry it, then sieve it, add magnesium stearate, then press the mixture into tablets, each tablet weighs 250mg, the active ingredient content 10mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com