SiRNA (Small interference ribonucleic acid) as well as medicine composition and pharmaceutical application thereof
A technology of small interfering nucleic acid and composition, applied in recombinant DNA technology, DNA/RNA fragments, etc., can solve the problems of poor biological activity and high cytotoxicity, and achieve the effect of inhibiting gene expression, reducing cytotoxicity and increasing serum stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] Optionally, the preparation method of the small interfering nucleic acid further comprises: phosphorothioate modification of the phosphodiester bond between the -3' terminal dTdT of the sense strand and the antisense strand.
[0060] Alternatively, a biotechnology company specializing in nucleic acid synthesis can also be entrusted to synthesize the siRNA of the present invention according to the nucleotide sequence shown in one of the above D1-D7, such as entrusting Shanghai GenePharma Company to synthesize.
[0061] Generally, the method for synthesizing small interfering nucleic acids includes the following four processes: (1) synthesis of oligoribonucleotides; (2) deprotection; (3) purification and separation; (4) desalting.
[0062] For example, the specific steps are as follows:
[0063] (1) Synthesis of oligoribonucleotides: set synthetic 1 millimolar RNA on an automatic DNA / RNA synthesizer (for example, AppliedBiosystems EXPEDITE8909), and set the coupling time ...
Embodiment 1
[0079] Entrust Shanghai GenePharma Technology Co., Ltd. (GenePharma) to synthesize small interfering nucleic acids with one of the modifications shown in D1-D7 below.
[0080] Table 1
[0081]
[0082]
Embodiment 2
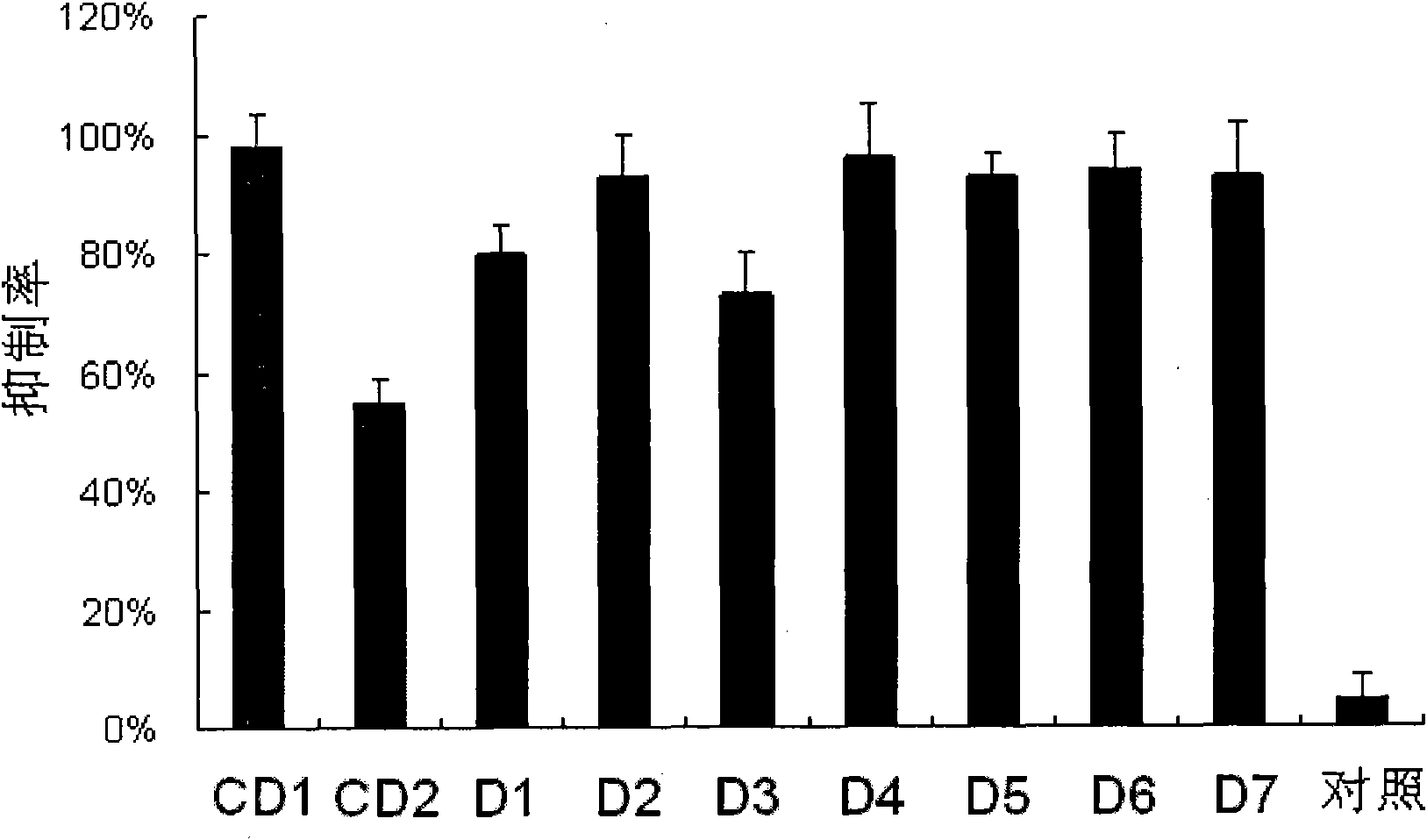
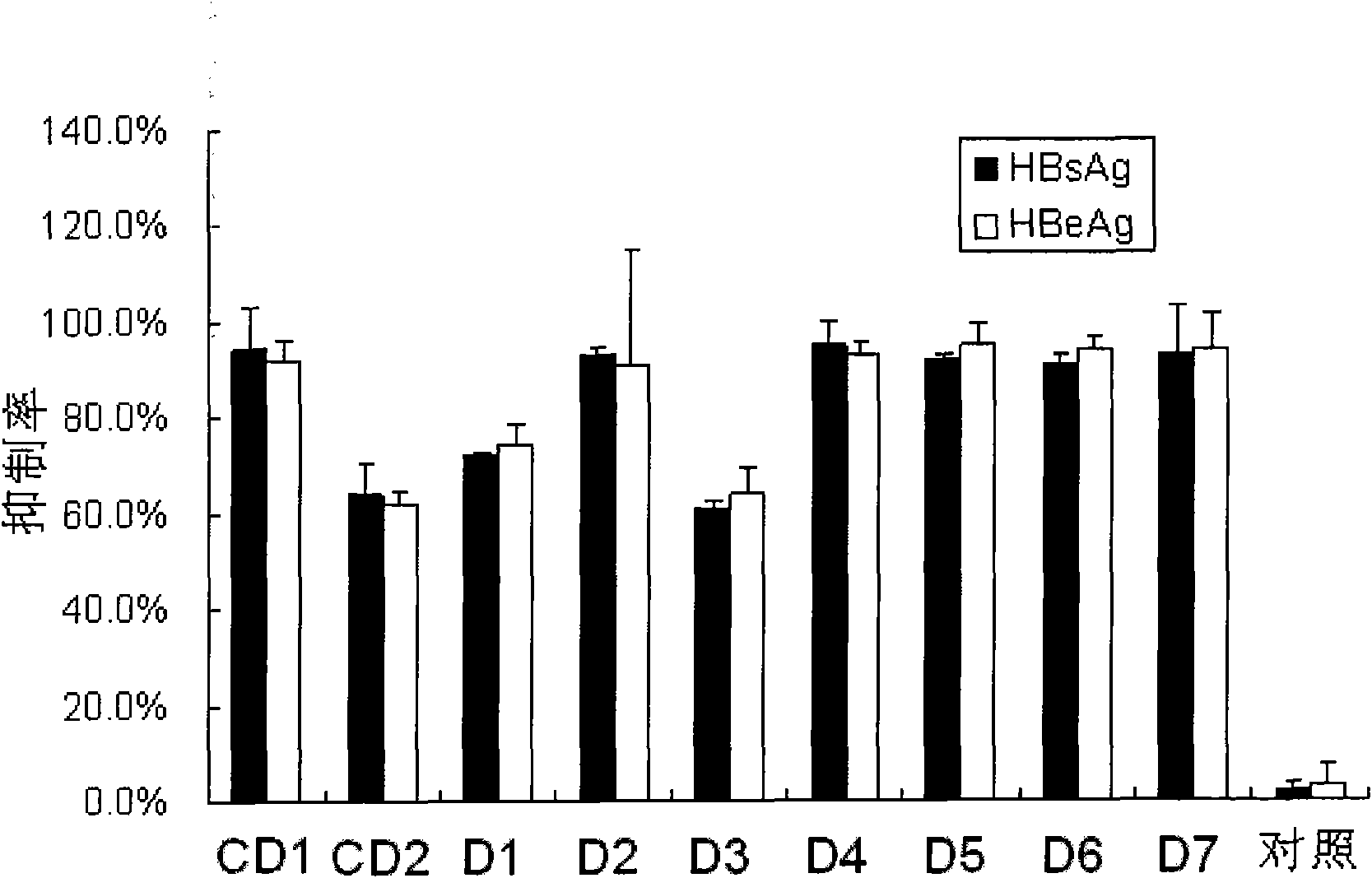
[0084] The small interfering nucleic acids with one of the modifications shown in D1-D7 are respectively detected as follows:
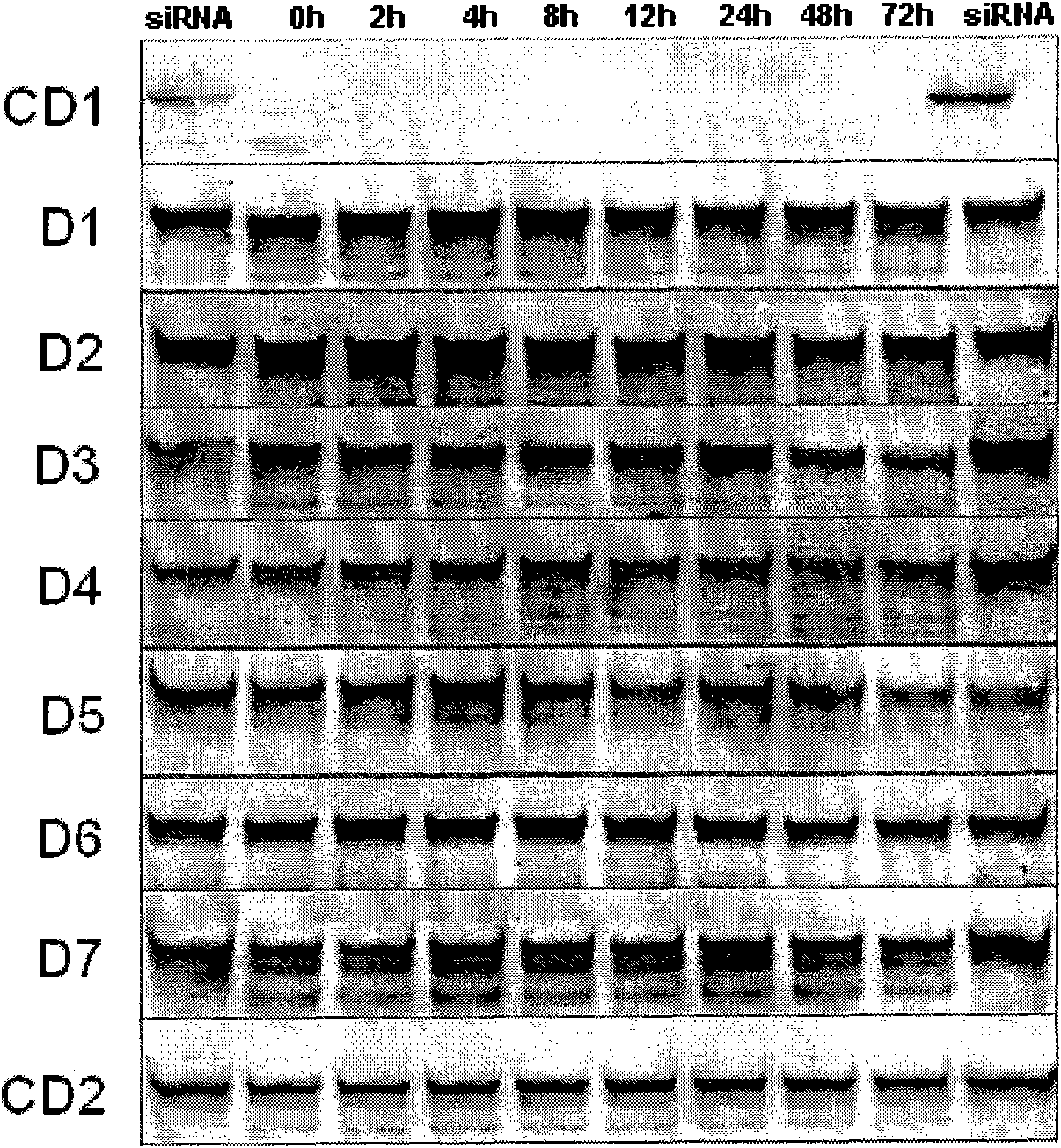
[0085] 1. Serum stability test
[0086] Add 10 μL of small interfering nucleic acid with a concentration of 20 μM to a solution (pH 7.4) containing 10 μL of fetal bovine serum and 80 μL of 1×PBS, incubate the reaction system at 37°C for a certain period of time and then take samples, respectively 0, 2, 4, 6, 8, 10, 12, 24 and 48 hours; take 10 μL samples each time and perform quick freezing in liquid nitrogen to immediately stop the effect of serum nucleases on small interfering nucleic acids, and then store the samples at -80°C for later use .
[0087] Configure a 20% by weight polyacrylamide gel, mix 3 μL of loading buffer (20mM EDTA, 36% by weight of glycerol, 0.06% by weight of bromophenol blue) with the degraded sample of siRNA, and then load the sample at 80mA Electrophoresis was performed under constant current conditions for 60 minutes. Aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com