Antitumor polypeptide and application thereof
An anti-tumor and anti-tumor drug technology, applied in the field of anti-tumor drug research and development and application, can solve the problem of low anti-cancer activity of 14 peptides, and achieve the effect of easy manufacture and significant anti-cancer effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The first polypeptide provided by the present invention is shown in SEQ ID NO: 1, which consists of 85 amino acids. 对应人类低氧诱导因子-1a第17位Arg至第101位Glu(85肽),ArgArgLysGluLysSerArgAspAlaAlaArgSerArgArgSerLysGluSerGluValPheTyrGluLeuAlaHisGlnLeuProLeuProHisAsnValSerSerHisLeuAspLysAlaSerValMetArgLeuThrIleSerTyrLeuArgValArgLysLeuLeuAspAlaGlyAspLeuAsp IleGluAspAspMetLysAlaGlnMetAsnCysPheTyrLeuLysAlaLeuAspGlyPheValMet,或者R R K E K S R D A A R S R R S K E S E V F Y E L A H QL P L P H N V S S H L D K A S V M R L T I S Y L R V RK L L D A G D L D I E D D M K A Q M N C F Y L K A L DG F V M(SEQ ID NO:1)
[0045] The polypeptide further provided by the present invention is a polypeptide consisting of at least 15 amino acids formed by consecutively reducing one or more amino acids from the C-terminus of the amino acid sequence shown in SEQ ID NO:1. For example:
[0046] 15 peptides: corresponding to the 17th Arg to ...
Embodiment 2
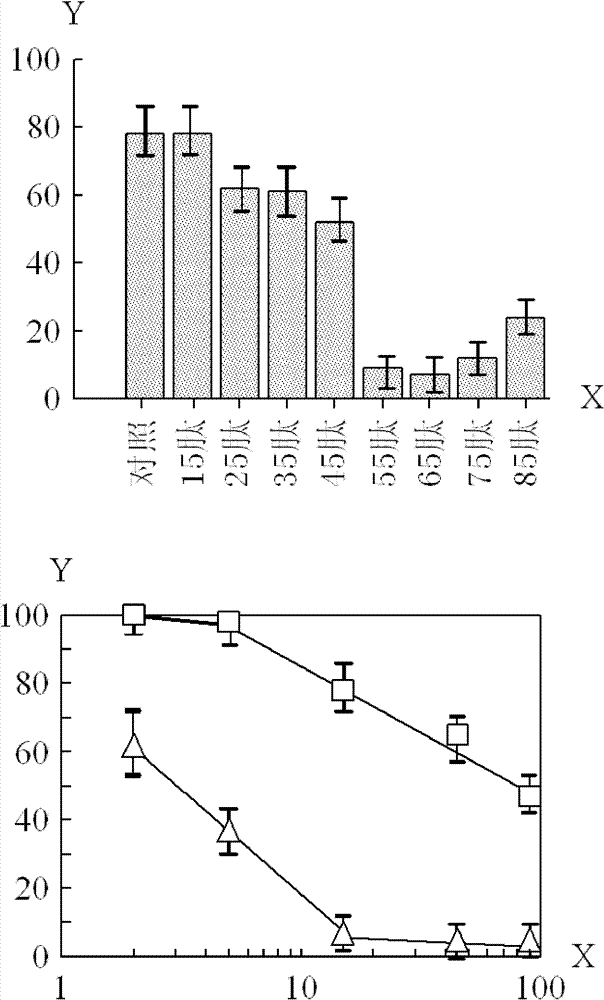
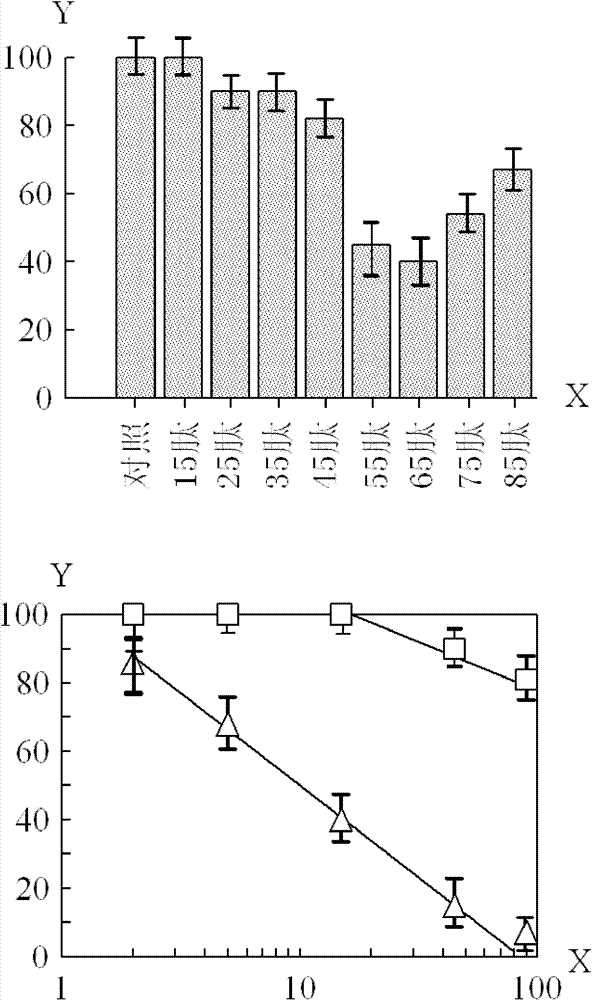
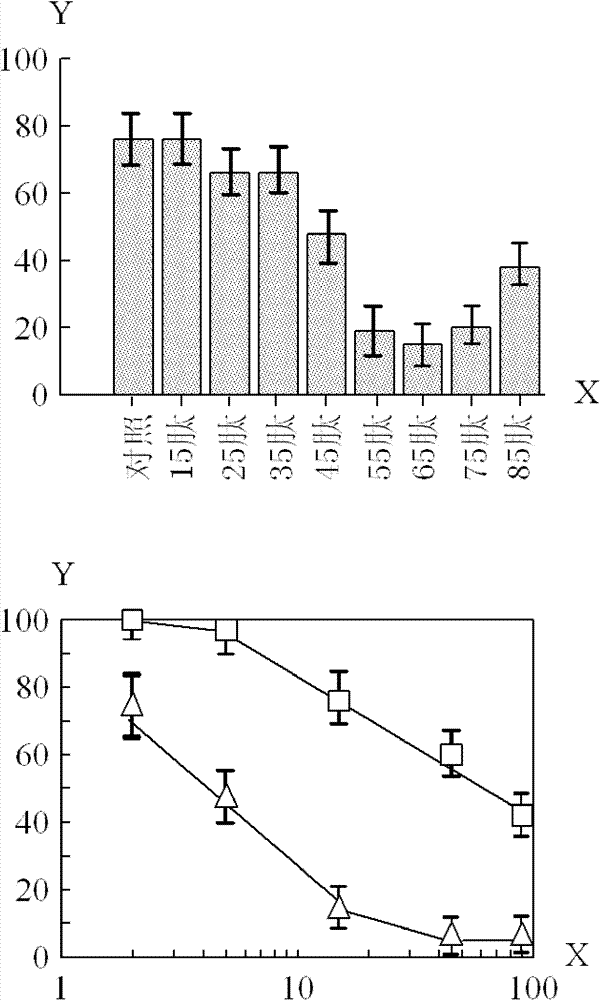
[0073] Usually, in order to reduce the production cost of the polypeptide, try to choose to produce a relatively short polypeptide on the premise of maintaining a relatively strong biological activity. Based on the above results, it was found that the 65 peptide was more effective in inhibiting tumor cell growth than the 45 peptide.
[0074] The difference between the 65 peptide and the 45 peptide is that the C-terminus of the 65 peptide has more sequence LeuThrIleSerLeuArgValArgLysLeuLeuAlaGlyAspLeuAspIleGlu than that of the 45 peptide. The length of this sequence is 20 amino acids, and it is this sequence that greatly increases the anti-tumor effect.
[0075] In order to identify the shortest effective length from the above 20 amino acid sequences, the following peptides were synthesized with the above 45 peptide (SEQ ID NO: 5) as the basic structure, adding 1-20 amino acids of varying numbers at the C-terminus:
[0076] 46 peptides:
[0077] ArgArgLysGluLysSerArgAspAlaAlaA...
Embodiment 3
[0121] In order to enhance the anti-tumor activity of the polypeptide, taking the 65 peptide with the strongest anti-tumor effect as an example, the following polypeptides were re-synthesized. They are based on 65 peptides and add sequences at the N-terminus and C-terminus respectively.
[0122] LysLysLysIl eSerSerGlu-65 Peptide:
[0123] LysLysLysI leSerSerGlu-ArgArgLysGluLysSerArgAspAlaAlaArgSerArgArgSerLysGluSerGluValPheTyrGluLeuAlaHisGlnLeuProLeuProHisAsnValSerSerHisLeuAspLysAlaSerValMetArgLeuThrI leSerTyrLeuArgValArgLysLeuLeuAspAlaGlyAspLeuAspIleGlu,或者K K K I S S E-R R K E K S R D A A R S R R S K E S E VF Y E L A H Q L P L P H N V S S H L D K A S V M R L TI S Y L R V R K L L D A G D L D I E(SEQ ID NO:27)
[0124] Corresponding to the 10th Lys to 16th Glu+65 peptide (17th Arg to 81st Glu) of human hypoxia inducible factor-1a protein.
[0125] 65 Peptide-GlnArgLysArgLysMet:
[0126]ArgArgLysGluLysSerArgAspAlaAlaArgSe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com