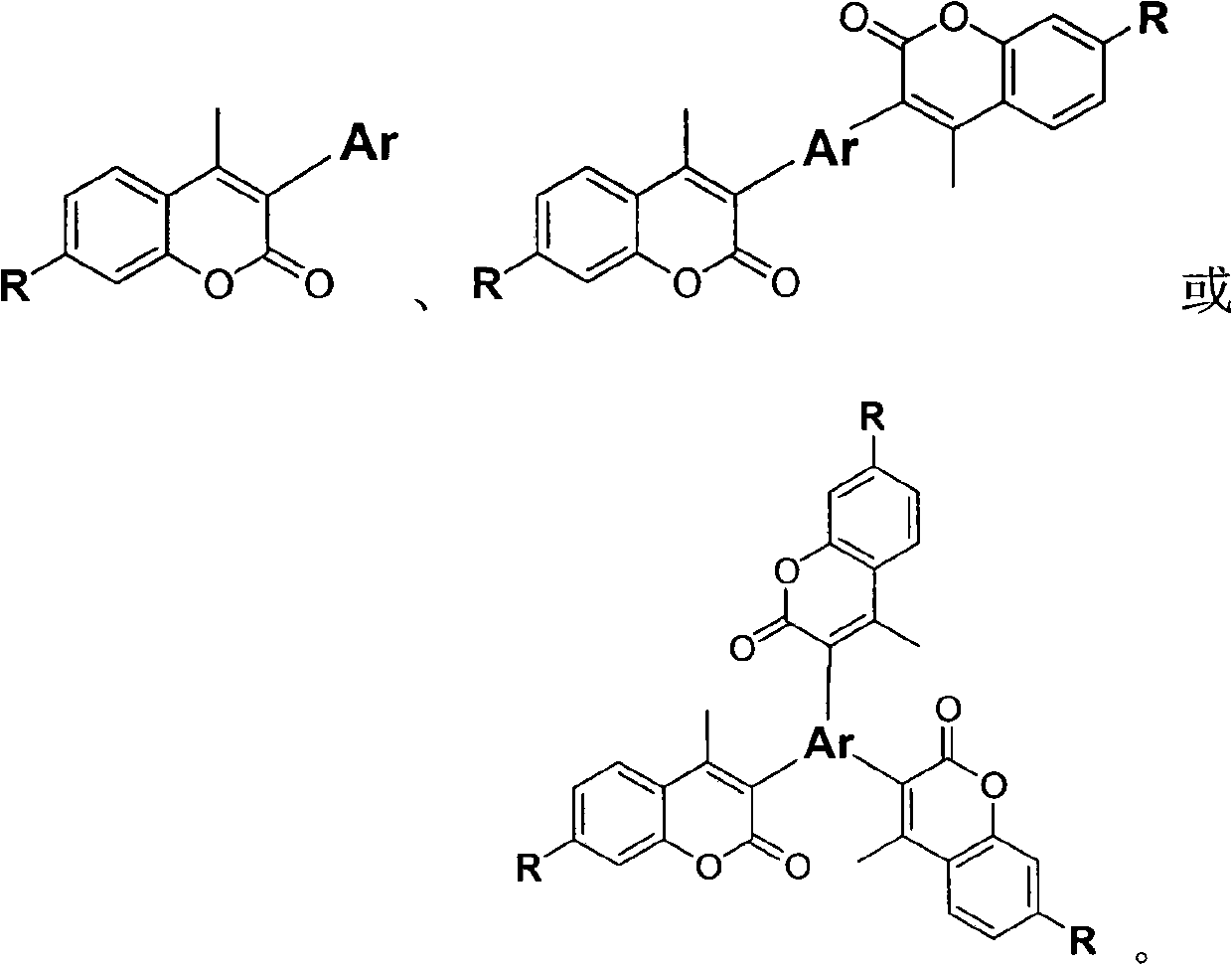
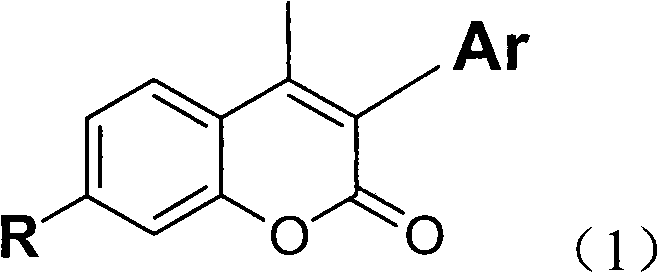
Coumarin derivates, preparation method thereof and application thereof in white-light organic electroluminescent device
A coumarin derivative, selected technology, applied in the direction of electric solid-state devices, electrical components, luminescent materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1. Synthesis of compound B1, synthetic route:
[0062]
[0063] Synthesis of intermediate M1: Add 7-ethoxy-4-methylcoumarin (2.04g, 10mmol), succinimide (abbreviated as NBS) (2.67g, 15mmol) and benzyl peroxide in a two-neck flask Acyl (referred to as DBP) (20mg) and chloroform (100mL), refluxed for 5 hours. After the reaction was completed, the chloroform layer was concentrated under reduced pressure, and the precipitate was washed with 500 mL of hot water (at a temperature of 50 to 80° C.) (to remove succinimide), filtered, and the resulting crude product was washed with a silica gel column (dichloromethane as a rinse). agent) was isolated and dried in vacuo to obtain intermediate M1 (2.58 g, 9.1 mmol, yield 91%).
[0064] Synthesis of compound B1: under nitrogen protection, add M1 (283mg, 1mmol), 4-triphenylamine borate (289mg, 1mmol), Pd(PPh 3 ) 4(92mg, 0.08mmol), 2M sodium carbonate (3mL), toluene (6mL) and ethanol (2mL), heated to reflux at 115°C f...
Embodiment 2
[0068] The synthesis of embodiment 2. compound B4, synthetic route:
[0069]
[0070] Synthesis of compound B4: under nitrogen protection, intermediate M1 (623mg, 2.2mmol) of Example 1, 1,4-benzenediboronic acid (166mg, 1mmol), Pd(PPh 3 ) 4 (92mg, 0.08mmol), 2M sodium carbonate (3mL), toluene (6mL) and ethanol (2mL), heated to reflux at 115°C for 24 hours. The reaction solution was extracted with chloroform, the extracted product was washed with water, dried over anhydrous magnesium sulfate, and the chloroform solvent was evaporated under reduced pressure. The obtained crude product was separated by a silica gel column (dichloromethane:methanol volume ratio=100:1), and dried in vacuo to obtain a white compound B4 solid (410mg, 0.85mmol, yield 85%).
[0071] Product MS (m / e): 482, corresponding to: C 30 h 26 o 6 =482, proving that the compound is B4.
[0072] Fluorescence quantum yield Φ f =78% (with the fluorescence quantum yield Φ of coumarin-1 in ethyl acetate f =...
Embodiment 3
[0074] The synthesis of embodiment 3. compound B5, synthetic route:
[0075]
[0076] Synthesis of Compound B5: The method and reaction conditions were the same as those of Compound B4 in Synthesis Example 2, except that 1,4-benzenediboronic acid was replaced by 4,4'-biphenyldiboronic acid, and the final product was a white powder.
[0077] Product MS (m / e): 558, corresponding to: C 36 h 30 o 6 =558, proving that the compound is B5.
[0078] Fluorescence quantum yield Φ f =65% (with the fluorescence quantum yield Φ of coumarin-1 in ethyl acetate f = 99% as standard).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Maximum brightness | aaaaa | aaaaa |
| Maximum current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com