Process for purifying recombinant human Fc fusion pegylated interferon
A technology of interferon and process, applied in the field of purification process, can solve the problems of complex purification process, difficulty in amplification, complex purification process of fusion interferon, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1. Pilot purification study of Fc-IFN (experimental batch number: 090712)
[0056] 1. Experimental purpose: Based on the conditions of the small test in the purification laboratory, the integration and optimization of the pilot test will be carried out.
[0057] 2. Chromatographic conditions: see Table 5.
[0058] 3. Experimental materials:
[0059] Protein A Sepharose FF (2.6cm×15cm);
[0060] Q Sepharose FF (2.6cm×10cm);
[0061] 10K ultrafiltration module;
[0062] Solution A: 20mM sodium dihydrogen phosphate / disodium hydrogen phosphate (pH7.5)+0.1M NaCl;
[0063] Solution B: 20mM citric acid-trisodium citrate (pH3.5);
[0064] Solution C: 20mM sodium dihydrogen phosphate / disodium hydrogen phosphate (pH7.5);
[0065] Solution D: 20mM sodium dihydrogen phosphate / disodium hydrogen phosphate (pH7.5)+1M NaCl;
[0066] Solution E: 10mM CH3COONH4+0.15M NaCl.
[0067] 4. Results and analysis:
[0068] Chromatography and electrophoresis results are shown in ...
Embodiment 2
[0073] Example 2. Fc-IFN pilot purification research (experimental batch number: 090728)
[0074] 1. Experimental purpose: Based on the conditions of the small test in the purification laboratory, the integration and optimization of the pilot test will be carried out.
[0075] 2. Chromatographic conditions: see Table 5.
[0076] 3. Experimental materials: see Example 1.
[0077] Chromatography and electrophoresis results are shown in Figure 7 , 8 , 9, 10 and Table 7.
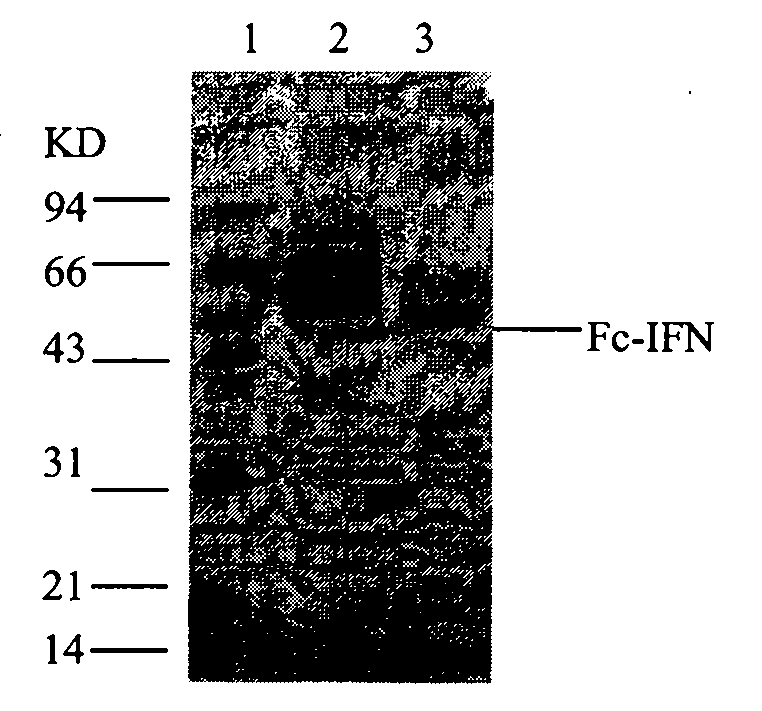
[0078] Figure 10 .Purified sample electrophoresis pattern (experimental batch number: 090728)
[0079] 1. Marker, 2. Cell culture medium, 3. G-25 chromatographic elution
[0080] Table 7: Analysis of Fc-IFN pilot purification study results (experimental batch number: 090728)
[0081]
Embodiment 3
[0082] Example 3. Fc-IFN pilot purification research (experimental batch number: 090814)
[0083] 1. Experimental purpose: Based on the conditions of the small test in the purification laboratory, the integration and optimization of the pilot test will be carried out.
[0084] 2. Chromatographic conditions: see Table 5.
[0085] 3. Experimental materials: see Example 1.
[0086] Chromatography and electrophoresis results are shown in Figure 11 , 12 , 13, 14 and Table 8.
[0087] Figure 14 .030814 electrophoresis pattern (experimental batch number: 090814)
[0088] 1.Marker, 2.G-25 chromatographic elution
[0089] Table 8: Analysis of Fc-IFN pilot purification research results (experimental batch number: 090814)
[0090]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com