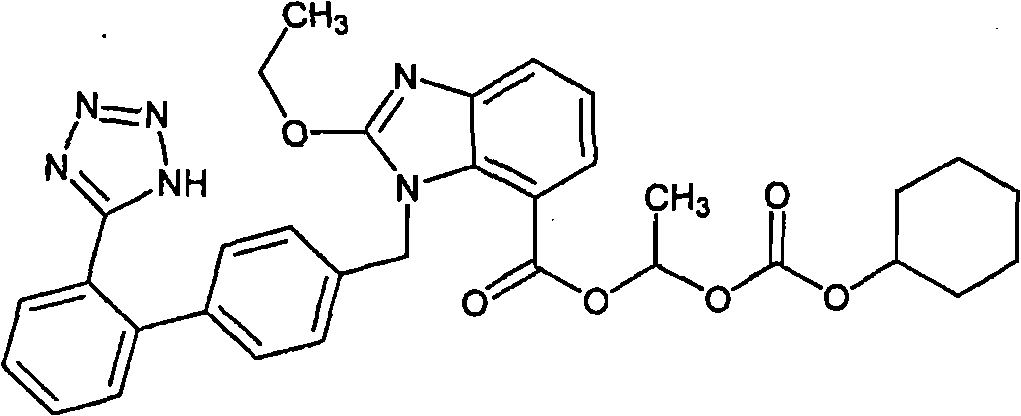
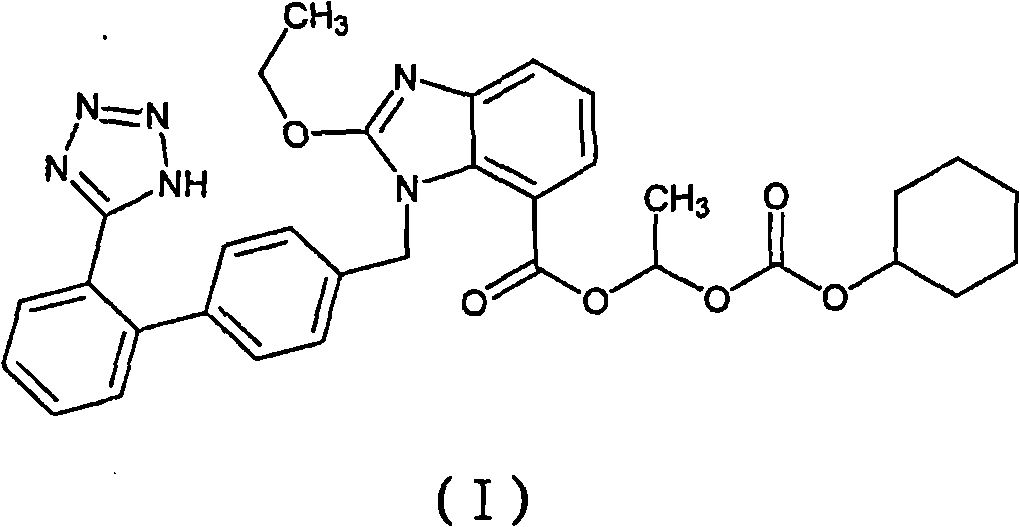
Candesartan cilexetil compound and novel preparation method thereof
A technology for candesartan medoxomil and a compound is applied in the refining field of candesartan medoxomil, and can solve the problems of unsatisfactory drug purity, low total yield, reduced content of active pharmaceutical ingredients, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Dissolve 100 g of candesartan cilexetil crude product with a purity of 97.3% in 1000 ml of acetonitrile, add 2 g of activated carbon, insulate at 50° C. and stir for 20 min, filter for decarburization, and collect the filtrate;
[0034] (2) Cool the above filtrate to room temperature, slowly add purified water, stir to produce a precipitate, and collect 95.1 g of the precipitate by filtration;
[0035] (3) Dissolve the above precipitate with 200ml of 1:1 petroleum ether (30-60°C) / ethyl acetate, add 200g of pretreated Sepabeads SP850 macroporous adsorption resin, and mix well to obtain the adsorbed candesartan cilexetil The resin is then added to the top of the Sepabeads SP850 type macroporous adsorption resin column, washed with purified water of 1 to 2 column volumes to clarification, then eluted with 30% ethanol eluent, and the eluted candesartan cilexetil is collected. The ethanol fraction was concentrated under reduced pressure and dried to obtain 92.5 g of refi...
Embodiment 2
[0037] (1) Dissolve 100 g of candesartan cilexetil crude product with a purity of 97.3% in 500 ml of acetone, add 1 g of activated carbon, stir at 50° C. for 10 min, filter for decarburization, and collect the filtrate;
[0038] (2) Cool the above-mentioned filtrate to room temperature, slowly add purified water, stir to produce a precipitate, and collect 94.8 g of the precipitate by filtration;
[0039] (3) Dissolve the above precipitate with 200ml of 1:1 petroleum ether (30-60°C) / ethyl acetate, add 200g of pretreated Sepabeads SP825901 type macroporous adsorption resin, and mix well to obtain the adsorbed candesartan cilexetil The resin is then added to the top of the Sepabeads SP825 type macroporous adsorption resin column, washed with purified water of 1 to 2 column volumes to clarification, then eluted with 30% ethanol eluent, and the eluted candesartan cilexetil is collected. The ethanol fraction was concentrated under reduced pressure and dried to obtain 90.1 g of refin...
Embodiment 3
[0041] (1) Dissolving 100 g of crude candesartan cilexetil with a purity of 97.3% in 2000 ml of methanol, adding 6 g of activated carbon, stirring at 50° C. for 30 min, filtering for decarburization, and collecting the filtrate;
[0042] (2) Cool the above-mentioned filtrate to room temperature, slowly add purified water, stir to produce a precipitate, and collect 94.4 g of the precipitate by filtration;
[0043] (3) Dissolving the above-mentioned precipitation with 200ml of 1:1 petroleum ether (30~60°C) / ethyl acetate, adding 200g of pretreated HPD300 type macroporous adsorption resin, mixing thoroughly to obtain the product of adsorbing candesartan cilexetil The resin is then added to the top of the HPD300 macroporous adsorption resin column, washed with 1 to 2 column volumes of purified water until clarified, then eluted with 30% ethanol eluent, and the ethanol part eluted by candesartan cilexetil is collected , concentrated under reduced pressure, and dried to obtain 88.4 g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com