New application of interleukin-12
A new application technology of interleukin, which is applied in the new application field of interleukin-12, can solve the problems of rejection, regulation disorder, testosterone propionate masculine side effects and other problems, and achieve good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
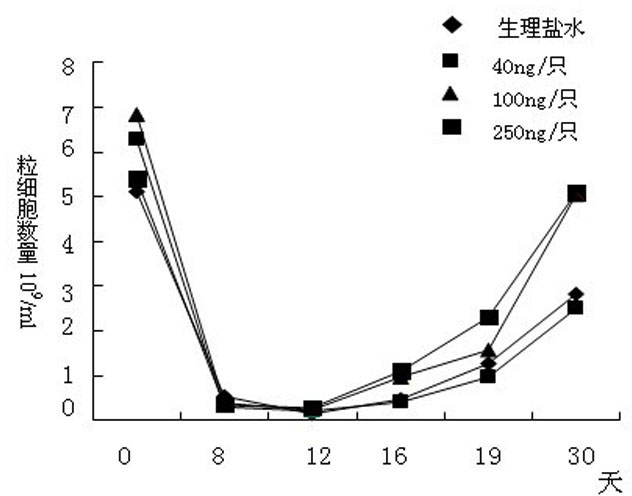
[0041] Expression of Apoptotic Rate of HSC in Mouse Bone Marrow Nucleated Cells Irradiated by Radiation
[0042] Purpose of the experiment: To observe the preventive and therapeutic effects of IL-12 on radiation-damaged cells
[0043] Experimental method: SPF grade Kunming mice were selected and irradiated with 4Gy by a linear accelerator. They were administered once 24 hours before radiation and 24 hours after radiation. They were sacrificed 4 days after irradiation, and the apoptosis rate of bone marrow HSC was detected. APC marked HSC, AnnexinⅤPE was the detection index of apoptosis.
[0044] (1) Preparation of bone marrow nucleated cells
[0045] BALB / c mice were killed by cervical dislocation, and bilateral femurs were taken under aseptic conditions, cultured in RPMI164 complete culture medium (with 10% fetal bovine serum added), and the bone marrow was obtained by repeatedly blowing and blowing with a syringe, and separated by lymphocyte separation medium Mononuclear c...
Embodiment 3
[0057] Preventive and Protective Effects of IL-12 on Physical Damage of Hematopoietic System Caused by Radiation
[0058] ①Protective effect of rmIL-12 on lethal dose radiation injury in mice
[0059] Test animals: 60 SPF grade BALB / c mice, 20-22 g, single sex.
[0060] Drugs used in the test: the negative control is sterilized normal saline, and the recombinant mouse interleukin-12 (rmIL-12) is the product of peprotech company.
[0061]
[0062] The administration methods were all subcutaneous administration, and the administration volume was 150 μl. The radiation method is whole body radiation, and the dose is 10Gy.
[0063] Observation indicators: Survival rate of mice 4 weeks after radiation.
[0064] Experimental results: All the mice in the first group died, 15 survived in the second group, and 13 survived in the third group.
[0065] Conclusion: Administration of rmIL-12 before and after lethal dose radiation can significantly improve the survival rate of mice. ...
Embodiment 4
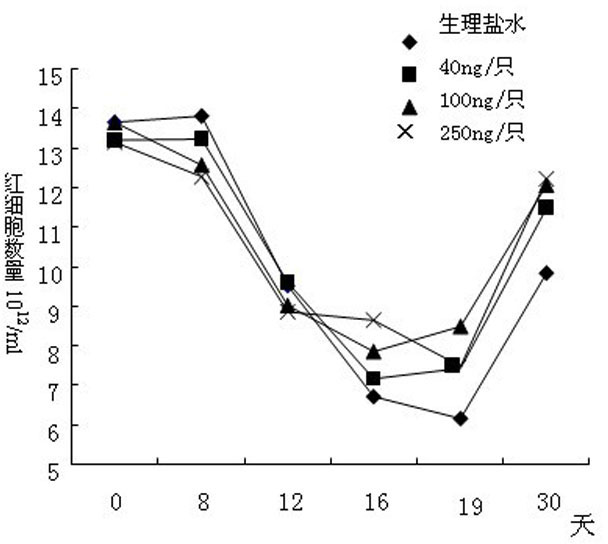
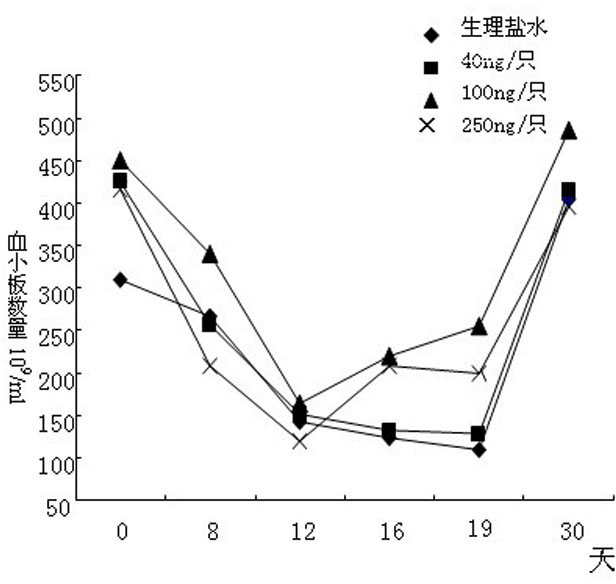
[0090] A test of the preventive and protective effects of rhIL-12 on the damage of the hematopoietic system caused by physical and chemical drugs.
[0091] Experimental plan: Use physical and chemical methods to injure the hematopoietic system of experimental mice, make a model of aplastic anemia, and then study the therapeutic effect of IL-12 on it.
[0092] experimental method:
[0093] Divide 100 Kunming rats into two groups. One group is the normal control group with 15 rats, 8 females and 7 males; the other group is the model group with 42 females and 43 males, totaling 85 rats. The model group was irradiated with 4Gy from a linear accelerator. On the fourth day, cyclophosphamide and chloramphenicol were administered for chemotherapy. The administration was continued for 3 days, once a day, and blood was collected on the 8th day for hemogram analysis. On the 16th day, the above combined regimen of radiation and chemotherapy was continued once again. In the model group, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com