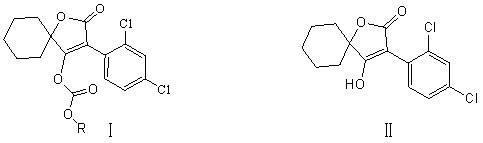
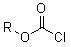
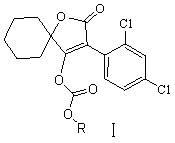
Novel spirodiclofen compound and preparation method and application thereof
A technology of ester compounds and compounds, applied in the field of pesticides, can solve the problems of the preparation method of new spirodiclofen compounds and the acaricidal activity that have not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1, a kind of synthetic method of novel spirodiclofen compound, its reaction is:
[0026]
[0027] Add 60ml of methylene chloride, 6.3g (0.02mol) of 3-(2,4-dichlorophenyl)-2-oxo Generation-1-oxaspiro[4,5]-dec-3-en-4-alcohol and 7.5g (0.075mol) triethylamine, after stirring and dissolving, add dropwise 3.3g (0.024mol) of sec-butyl chloroformate under ice-water bath mol), then continue to stir at room temperature, and the thin-layer plate controls the reaction time. After the reaction, the reaction solution was poured into saturated NaHCO 3 solution (about pH 8), stirred for ten minutes, separated, washed the organic layer with 40-60ml of water, and used anhydrous Na 2 SO 4 Dry, and remove the solvent methylene chloride under reduced pressure to obtain a light yellow viscous liquid, which is recrystallized with 50 ml of methanol to obtain 7.08 g of colorless crystals (namely I a in Table 1), yield: 86%. Melting point: 100~102℃.
[0028] 1 H NMR (500Hz, ...
Embodiment 2
[0029] Embodiment 2, a kind of synthetic method of novel spirodiclofen compound, its reaction is:
[0030]
[0031] Add 60ml 1,2-dichloroethane, 6.3g (0.02mol) 3-(2,4-dichlorophenyl )-2-oxo-1-oxaspiro[4,5]-dec-3-en-4-alcohol and 7.5g (0.075mol) triethylamine, after stirring and dissolving, add n-butyl chloroformate dropwise under ice-water bath 3.3 g (0.024 mol) of ester, and then continue stirring at room temperature, and the reaction time is controlled by a thin-layer plate. After the reaction, the reaction solution was poured into saturated NaHCO 3 solution (about pH 8), stirred for ten minutes, separated, washed the organic layer with 40-60ml of water, and used anhydrous Na 2 SO 4 Drying, removing the solvent 1,2-dichloromethane under reduced pressure to obtain a light yellow oily liquid, which was recrystallized with 50ml of methanol to obtain 6.59g of colorless crystals (i.e. Ib in Table 1), yield: 80%. Melting point: 92-93°C.
[0032] 1 H NMR (500Hz, CDCl 3 , ...
Embodiment 3
[0033] Embodiment 3, a kind of synthetic method of novel spirodiclofen compound, its reaction is:
[0034]
[0035] Add 60ml of benzene, 6.3g (0.02mol) 3-(2,4-dichlorophenyl)-2-oxo- 1-oxaspiro[4,5]-dec-3-en-4-alcohol and 5.9g (0.075mol) pyridine, after stirring and dissolving, 3.6g (0.024mol) of isoamyl chloroformate was added dropwise under ice-water bath, and then Stirring was continued at room temperature, and the reaction time was controlled by a TLC plate. After the reaction, the reaction solution was poured into saturated NaHCO 3 solution (about pH 8), stirred for ten minutes, separated, washed the organic layer with 40-60ml of water, and used anhydrous Na 2 SO 4 After drying, the solvent benzene was removed under reduced pressure to obtain a light yellow oily liquid, which was recrystallized with 50 ml of methanol to obtain 6.9 g of colorless crystals (ie Ic in Table 1), yield: 82%. Melting point: 95~96℃.
[0036] 1 H NMR (500Hz, CDCl 3 , δppm): 7.41~7.21 (3H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com