Effective cardiotonic part of erigeron breviscapus and preparation method thereof
An effective part and technology of breviscapus, applied to the effective part of breviscapine for cardiotonic effect and its preparation method and pharmaceutical preparations containing them, the application of chronic congestive heart failure drugs, the treatment of acute field, can solve the non-compliance and side effects Small and cardiac glycoside components Breviscapita can not be enriched and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
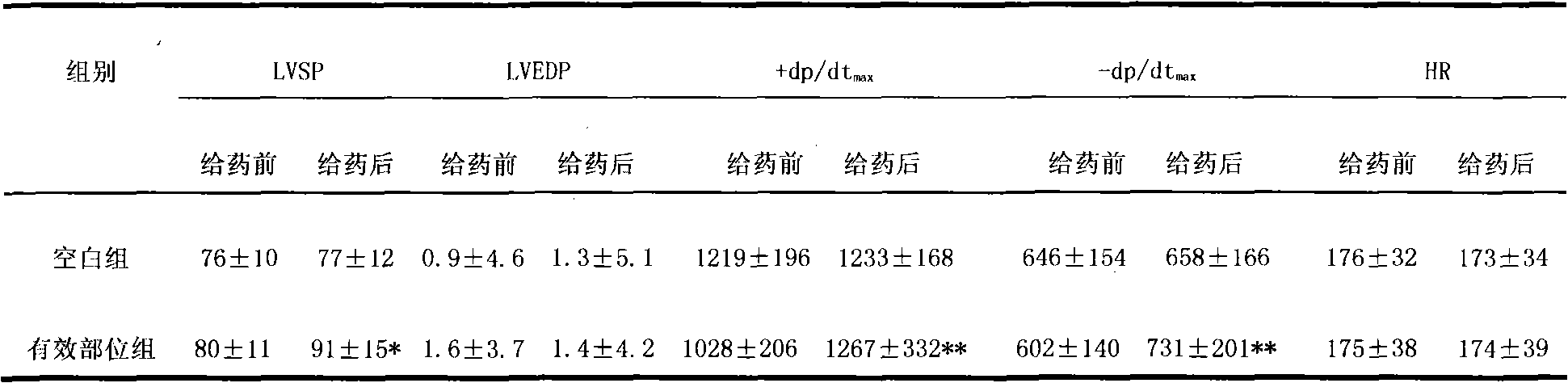
Image
Examples
Embodiment 1
[0027] Breviscapus extract 60g, suspended in water, filtered with D 101 Macroporous resin adsorption, first washed with water, then eluted with 10% ethanol, then eluted with 60% ethanol, collected 60% eluent, concentrated to dryness under reduced pressure, and recovered the solvent; the resulting solid was subjected to silica gel column chromatography, Ethyl acetate:methanol=20:1 isocratic elution, each 100mL as a portion, using thin-layer chromatography (developing solvent: chloroform:methanol:water=65:35:10 lower layer, silica gel G thin-layer plate, 3 , 5'-dinitrobenzoic acid reagent for color development) to check the fractions collected, the fractions containing scutellaria breviscapus were combined, concentrated under reduced pressure and then carried out a silica gel column chromatography under the same conditions, after thin layer inspection (thin Layer conditions are the same as above), the fractions with higher content of scutellaria breviscapus were combined, concen...
Embodiment 2
[0029]25g scutellaria extract, mix with 25g silica gel, carry out Soxhlet extraction with ethyl acetate, extract for 12 hours; the extract is concentrated to dryness under reduced pressure, recover the solvent, mix the obtained solid with 5g silica gel, and load the sample on silica gel column, first eluted with ethyl acetate, then eluted with ethyl acetate: ethanol = 25: 1, and tested by TLC (the TLC conditions are the same as in Example 1), the fractions with higher scutellaria content were combined and freeze-dried , to obtain 48 mg of the effective fraction of scutellaria breviscapus, and the content of scutellaria breviscapus was 58% as determined by HPLC; fractions with lower scutellaria scutellaria content could be re-combined for column chromatography.
Embodiment 3
[0031] Scutellaria breviscapus extract 5g carries out reverse-phase column chromatography, filler is carbon-octave bonded phase silica gel, uses 10%~99.5% methanol gradient elution, collects scutellaria breviscapus content through thin-layer inspection (thin-layer condition embodiment 1) The high fractions were combined, concentrated and then spray-dried to obtain 10 mg of the effective fraction of scutellaria breviscapus, which was determined by HPLC to have a content of 56%; the fractions with lower scutellaria content can be re-performed reversed-phase column layer analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com