Application of artemisinin derivative in preparation of medicaments for treating Crohn disease
A technology of artemisinin derivatives and Crohn's disease, applied in the field of biomedicine, can solve the problems of large side effects and unsatisfactory effects of Crohn's disease, and achieve the effect of less side effects, mild side effects and effective treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Animal grouping and establishment of colitis model
[0033] C57BL / 6 mice (supplied by Shanghai Experimental Animal Center, Chinese Academy of Sciences) were randomly divided into 3 groups, namely, ethanol control group, trinitrobenzenesulfonic acid model group and artesunate treatment group, with 6 mice in each group. Establishment of the mouse colitis model in the trinitrobenzenesulfonic acid group and the artesunate treatment group: After the mice were lightly anesthetized, the flexible tube was inserted into the anus about 4cm, and 0.1ml of trinitrobenzenesulfonic acid / ethanol solution was slowly injected; ethanol control The group was injected with 0.1ml of ethanol solution, inverted for about 60s, and put back into the cage. About 8 hours after the enema, the mice in the artesunate treatment group were intraperitoneally injected with artesunate 150 mg / kg / day (Guangxi Guilin Pharmaceutical Co., Ltd.), and the ethanol control group and the trinitrobenze...
Embodiment 2
[0036] Example 2: Histological examination
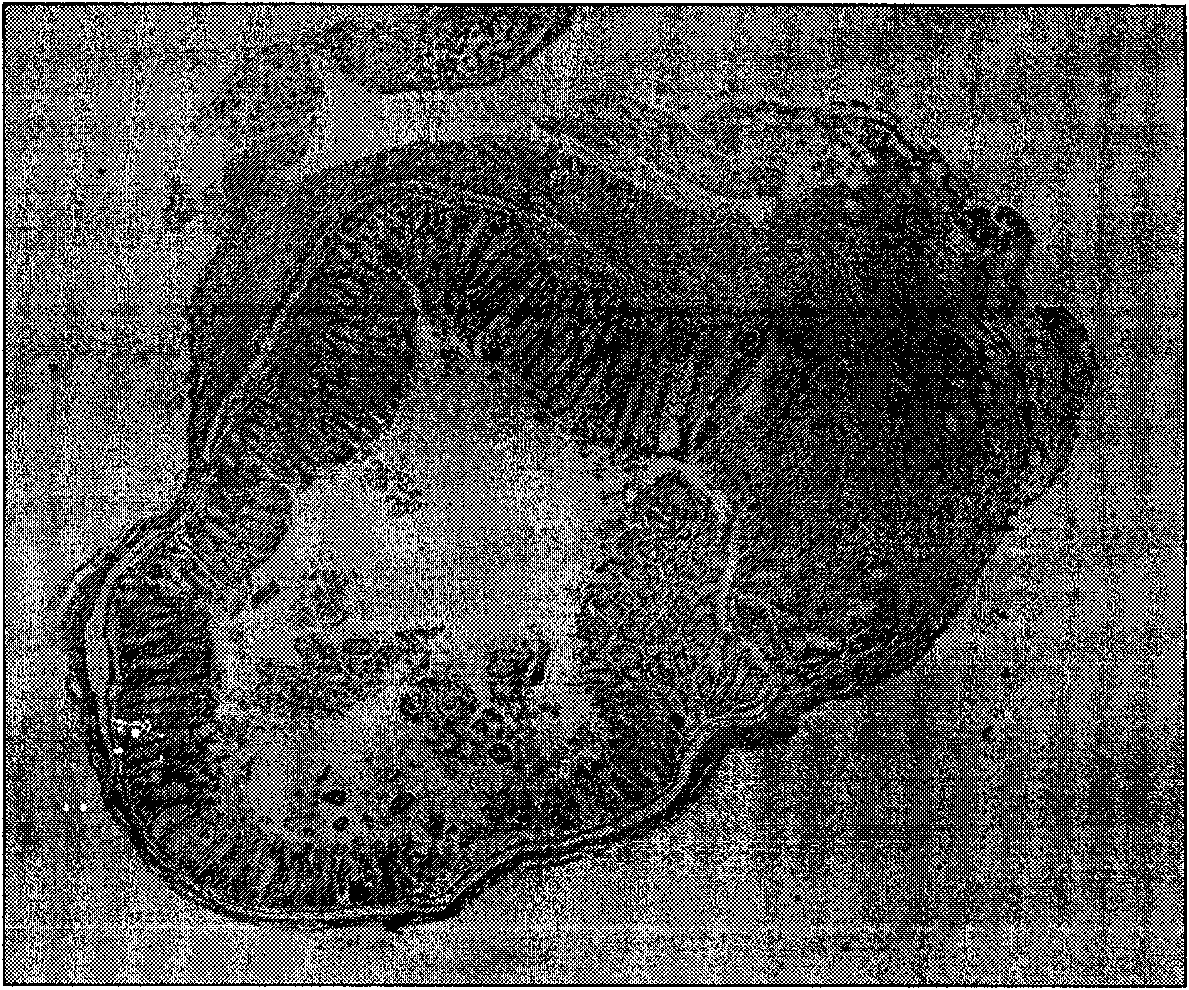
[0037] On the eighth day of the experiment, the mice were sacrificed, and the serum was collected and stored in a -70°C refrigerator; laparotomy was performed, and the intestines were collected and stored in liquid nitrogen. Colonic changes were observed with the naked eye and a general score was made. The criteria were: colon ulcer, colon and peritoneal adhesions, colon wall thickening, and mucosal edema. The severity of each parameter ranged from normal (0 points) to the most severe (4 points) points 4 The general score is the average of the four parameter scores. Colon tissues with the most obvious lesions were taken and fixed in 10% formalin solution, routinely embedded in paraffin, sectioned, stained with hematoxylin-eosin, observed histological changes with a light microscope and scored, and the standard was: 0 points: no inflammation; 1 point : mild inflammation of the colon; 2 points: a small amount of inflammatory cells su...
Embodiment 3
[0038] Example 3 Immunohistochemical staining and result judgment
[0039] The SP method was used for nuclear factor-κBp65 immunohistochemical staining, and the specific operation steps were carried out according to the instructions of the nuclear factor-κBp65 SP immunohistochemical staining kit (Fujian Maixin Biotechnology Development Co., Ltd.). The result judgment includes two aspects: (1) Positive staining intensity grading: level 0 is negative, no cell staining; level 1 is weakly positive, scattered yellow particles appear in the cells; level 2 is moderately positive, a small amount of brown-yellow cells appear Particles; grade 3: a large number of brown-yellow particles appear in the cells. (2) Grading of the number of positive cells: Grade 0 is negative, no cell staining; Grade 1 is the number of positive cells ≤ 10%; Grade 2 is the number of positive cells 11% to 50%; Grade 3 is the number of positive cells 51% to 80% ; Grade 4 is the number of positive cells > 80%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com