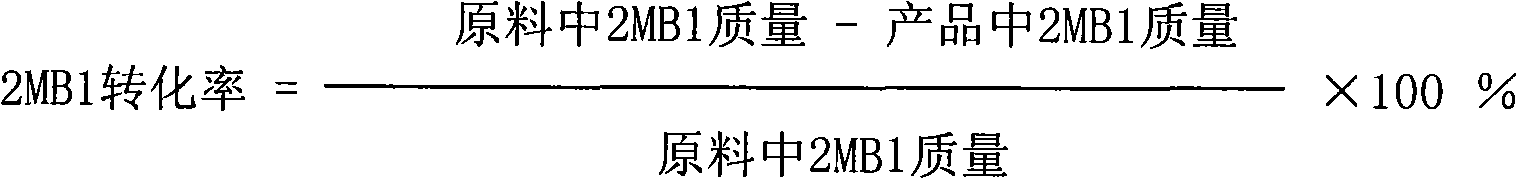
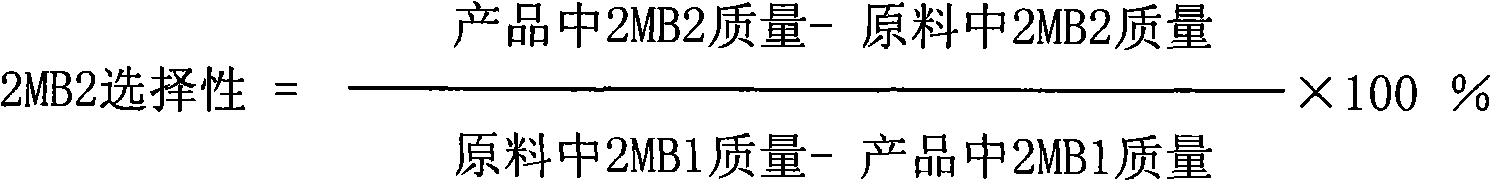
Method for preparing isoamylene by using tertiary amyl methyl ether
A technology of methyl tert-amyl ether and isopentene, which is applied in the field of isopentene from methyl tert-amyl ether, can solve problems such as increasing the burden of product purification, eliminate abnormal fluctuations in catalyst bed temperature, overcome defects, The effect of reducing content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~10
[0024] 1. Thermal cracking of methyl tert-amyl ether
[0025] The raw material for the reaction is the methyl tert-amyl ether obtained by reacting the by-product C5 fraction produced by steam cracking of naphtha to ethylene and methanol. After methyl tert-amyl ether is heated and vaporized, the α-Al is modified by HF in the gas phase. 2 o 3 The packed catalyst bed carries out etherolysis reaction, and the reaction temperature is controlled at 180-230°C.
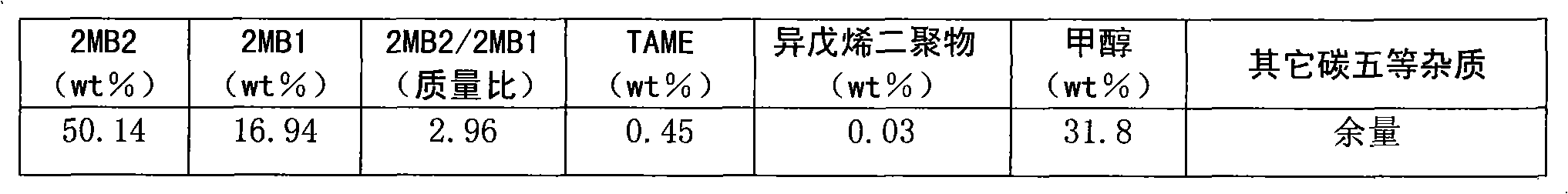
[0026] The main composition of the etherolysis products is shown in Table 1.
[0027] Table 1.
[0028]
[0029] 2. Isomerization reaction
[0030] The reaction is carried out in a φ25mm×1500mm stainless steel tubular reactor, and a temperature-controlled jacket of circulating hot water is installed outside the reactor. Catalysts are filled in the reactor to form a fixed-bed catalyst bed, and temperature-measuring platinum resistors are respectively installed on the top, middle and bottom of the catalyst bed. The cat...
Embodiment 11~15
[0043] The isopentene obtained in Examples 1-10 is mixed and then rectified and refined. The rectification tower is a packed tower with 40 theoretical plates. Refined isopentene is produced from the side line, and light components and heavy components are discharged from the top and bottom of the tower respectively. The specific process conditions and the purity of the refined isoamylene product are shown in Table 4.
[0044] Table 4.
[0045]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com