6,7-methylene-dioxy-1,2,3,4-tetrahydroisoquinoline derivative and preparation method and application thereof
A methylenedioxy, tetrahydroisoquinoline technology, applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
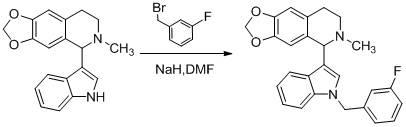
[0104] Example 1: 5-[1-(2-chlorobenzyl)-3-indolyl]-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxa[4 ,5-g] Preparation of Isoquinoline (Compound 1)
[0105] 1) Preparation of 1,2-methylenedioxy-4-(2-nitro-vinyl)-benzene
[0106] Add 17.5g of piperonal (0.117mol) and 7.63g of ammonium acetate (0.096mol) into 100ml of glacial acetic acid, add dropwise 18.2ml of nitromethane, react at 105°C for 6h, and cool to room temperature. Suction filtration, washing the filter cake with a small amount of cold ethanol, and drying. 17.1 g was obtained, and the yield was 76%. Melting point: 161-163°C.
[0107] 2) Preparation of 2-(1,2-methylenedioxy-4-phenyl)-ethylamine hydrochloride
[0108] Under the condition of ice bath, 18g of lithium aluminum hydride (0.5mol) was dissolved in 800ml of dry tetrahydrofuran solution, and the reaction temperature was kept below 10°C. Slowly put 22.3g (0.1mol) of 1,2-methylenedioxy-4-(2-nitro-vinyl)-benzene into the reaction solution and stir at room temperature ...
Embodiment 2
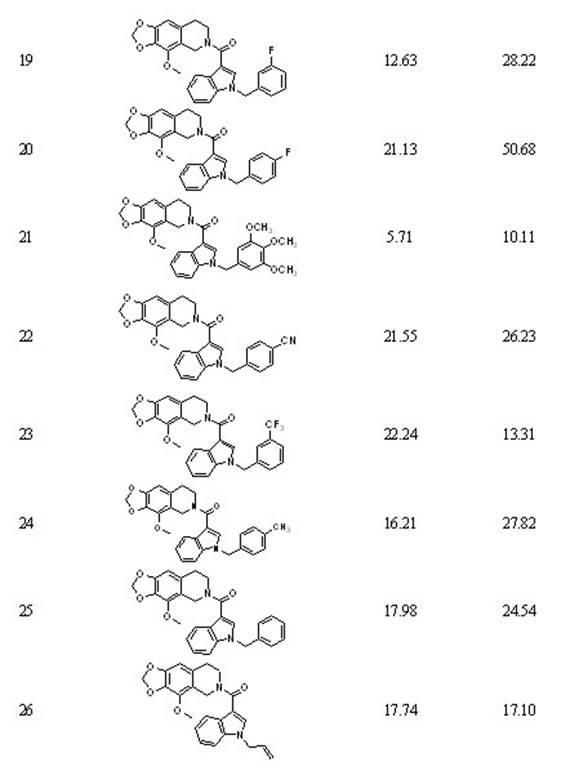
[0119] Embodiment two: 5-[1-(3 -Chlorobenzyl ) - 3 -indolyl ]-6- methyl -5,6,7,8- Tetrahydro- [1,3] Dioxa [4,5-g] Preparation of isoquinoline (compound 2)
[0120] 5-(3- Indolyl )-6- methyl -5,6,7,8- Tetrahydro -[1,3] Dioxa [4,5-g] Isoquinoline reacts with 3-chlorobromobenzyl, the same operation 1 Preparation, yield: quantitative. 1 H-NMR (300MHz, CDCl 3 , ppm) δ: 2.35(s, 3H), 2.72(t, 1H), 2.85(t, 1H), 3.18(q, 2H), 4.69(s, 1H), 5.27(s, 2H), 5.80(d , 2H), 6.33(s, 2H), 6.60(s, 1H), 6.92(d, 1H), 7.04(m, 4H), 7.10(m, 1H), 7.22(s, 2H), 7.47(d, 1H, J =7.8); ESI-MS: m / z 431.1 [M+H] + .
Embodiment 3
[0121] Embodiment three: 5-[1-(4 -Chlorobenzyl )-3 -indolyl ]-6- methyl -5,6,7,8- Tetrahydro -[1,3] Dioxa [4,5-g] Preparation of isoquinoline (compound 3)
[0122] Depend on 5-(3- Indolyl )-6- methyl -5,6,7,8- Tetrahydro -[1,3] Dioxa [4,5-g] Prepared by reacting isoquinoline with 4-chlorobromobenzyl, the same operation 1 Preparation, yield: quantitative. 1 H-NMR (600MHz, CDCl 3 , ppm) δ: 2.21(s, 3H), 2.52(t, 1H), 2.72(t, 1H), 3.05(m, 2H), 4.43(s, 1H), 5.18(s, 2H), 5.72(d , 2H), 6.25(s, 1H), 6.52(s, 1H), 6.94(q, 1H), 7.07(t, 1H), 7.11(t, 1H), 7.18(t, 2H), 7.42(d, 1H, J =3.9); ESI-MS: m / z 431.2 [M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com