Method for preparing 7-amino-3-nor-3-cephalo-4-carboxylic acid
A cephalosporin and amino technology, which is applied in the field of preparation of 7-amino-3-non-3-cephalosporin-4-carboxylic acid, can solve the problems of high metal ion residue, harsh use conditions, and poor product appearance, and achieve technological Simple and easy to implement, reduce production costs and improve product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
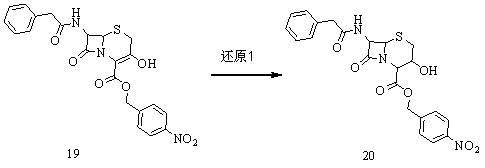
[0050] Weigh 46.9g of 3-hydroxycephalosporin in a 500ml three-neck flask (with thermometer and mechanical stirring) 19 (0.1mol), add 200ml tetrahydrofuran to dissolve. The temperature was lowered to -30°C, and 5.4 g of potassium borohydride (0.1 mol) was added. Continue to cool down to -60°C, and slowly add 100ml of ethanol dropwise. After the dropwise addition, continue to react for 1 hour, take samples for HPLC detection, and control the reaction raw materials 19 Content≤0.5%. 400ml of purified water was added, the temperature was slowly raised to room temperature, and a solid was precipitated. The tetrahydrofuran solvent was recovered under reduced pressure at room temperature. Filter and dry to get 42.39g white solid product 20 (The molar yield is 90%, and the HPLC area normalization method content is 98%).
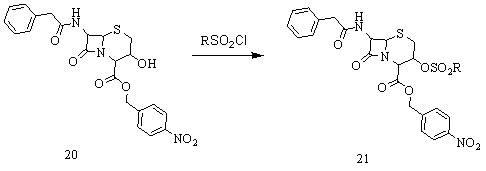
[0051] Add 42.3g of the above product in a 1000ml three-necked bottle 20 (0.09mol), add 300ml tetrahydrofuran to dissolve. Cool down to -15°C, add 13.7 g ...
Embodiment 2
[0058] Weigh 46.9g of 3-hydroxycephalosporin in a 500ml three-neck flask (with thermometer and mechanical stirring) 19 (0.1mol), add 200ml of dichloromethane to dissolve. The temperature was lowered to -30°C, and 4.18 g of sodium borohydride (0.11 mol) was added. Continue to cool down to -55°C, and slowly add 100ml of methanol dropwise. After the dropwise addition, continue to react for 1 hour, take samples for HPLC detection, and control the reaction raw materials 19 Content≤0.5%. 400ml of purified water was added, the temperature was slowly raised to room temperature, and a solid was precipitated. The dichloromethane and methanol solvents were recovered under reduced pressure at room temperature. Filter and dry to get 41.5g white solid product 20 (The molar yield is 88%, and the HPLC area normalization method content is 98.5%).
[0059] Add 41.4g of the above product in a 1000ml three-necked bottle 20 (0.088mol), add 350ml tetrahydrofuran to dissolve. Cool down ...
Embodiment 3
[0062] Weigh 46.9g of 3-hydroxycephalosporin in a 500ml three-neck flask (with thermometer and mechanical stirring) 19 (0.1mol), add 100ml of dichloromethane and 100ml of tetrahydrofuran to dissolve. The temperature was lowered to -30°C, and 5.4 g of potassium borohydride (0.1 mol) was added (0.11 mol). Continue to cool down to -60°C, and slowly add 100ml of ethanol dropwise. After the dropwise addition, continue to react for 1 hour, take samples for HPLC detection, and control the reaction raw materials 19 Content≤0.5%. 400ml of purified water was added, the temperature was slowly raised to room temperature, and a solid was precipitated. The dichloromethane and tetrahydrofuran solvents were recovered under reduced pressure at room temperature. Filter and dry to get 43g white solid 20 (The molar yield is 91.3%, and the HPLC area normalization method content is 98.7%).
[0063] Add 43g of the above product in a 1000ml three-necked bottle 20 (0.0913mol), 300ml of 1,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com