Curcumin coated liposome preparation and preparation method thereof
A technology of liposome preparation and curcumin, which is applied in the field of medical applications, can solve the problems of low oral bioavailability, low absorption, poor fat solubility, etc., and achieve the effects of promoting dissolution and absorption, improving stability, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
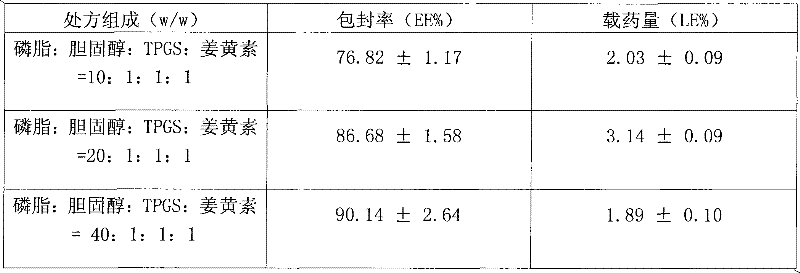
[0036] Accurately weigh 10mg curcumin, 100mg soybean lecithin, 10mg cholesterol, add to 20ml absolute ethanol, remove ethanol under reduced pressure at 40°C, dry overnight in a vacuum oven, add 20ml phosphate buffer saline (PBS, pH 5~ 7) Carry out hydration, centrifuge to remove the decrystallization drug, filter the obtained liposome suspension A through a 0.8 μm microporous membrane, homogenize, and drop the obtained liposome suspension B into the TMC solution In C (0.1%, w / v), incubate for 1 h to obtain curcumin-coated liposome suspension D of the present invention.
[0037] The encapsulation efficiency of the obtained liposome was 9.21%, and the drug loading capacity was 0.445%.
Embodiment 2
[0039]Accurately weigh 10 mg of curcumin, 200 mg of soybean lecithin, and 10 mg of cholesterol, add them to 30 ml of absolute ethanol, remove the ethanol by rotary evaporation under reduced pressure at 40 ° C, dry in a vacuum oven overnight, add 20 ml of phosphate buffer saline (PBS, pH 5-7) Carry out hydration, centrifuge to remove the crystalline drug, filter the obtained liposome suspension A through a 0.8 μm microporous membrane, homogenize, and drop the obtained liposome suspension B into the TMC In solution C (0.2%, w / v), incubate for 1 h to obtain curcumin-coated liposome suspension D of the present invention. Take 2ml of the prepared suspension D, add mannitol (1%, w / v), put it into a vial, pre-freeze in the refrigerator at -80°C for 24h, and then put it in a freeze dryer at -40°C, 0.10mbar for 48h A lyophilized preparation is obtained.
[0040] Take an appropriate amount of the suspension, drop it on the copper grid, carry out negative staining with 2% phosphotungsti...
Embodiment 3
[0042] Accurately weigh 10mg of curcumin, 400mg of soybean lecithin, 20mg of Pluronic F127 (PF127), add to 20ml of ethyl acetate, remove the organic solvent by rotary evaporation under reduced pressure at 40°C, dry in a vacuum oven overnight, add 20ml of phosphoric acid Salt buffer solution (PBS, pH 5-7) was hydrated, centrifuged to remove crystalline drug, and the obtained liposome suspension A was filtered through a 0.8 μm microporous membrane, homogenized, and the obtained liposome suspension was B was added dropwise into TMC solution C (0.5%, w / v), and incubated for 2 hours to obtain curcumin-coated liposome suspension D of the present invention. Take 2ml of the prepared suspension D, add sucrose (4%, w / v), put it into a vial, pre-freeze in the refrigerator at -80°C for 24h, and then put it in a freeze dryer at -40°C, 0.10mbar for 48h A lyophilized preparation is obtained.
[0043] The zeta potential measurement results showed that the coated liposome was +15.64mV, and th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com