Combination method for increasing yield of p-xylene in aromatic hydrocarbon production
The technology of paraxylene and combination method is applied in the combined field of increasing paraxylene production in aromatic hydrocarbon production, and can solve the problems of high energy consumption, large circulation volume of xylene separation unit and isomerization unit, low concentration and the like, and achieve adsorption Separation device simplification, flexibility, and concentration enhancement effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
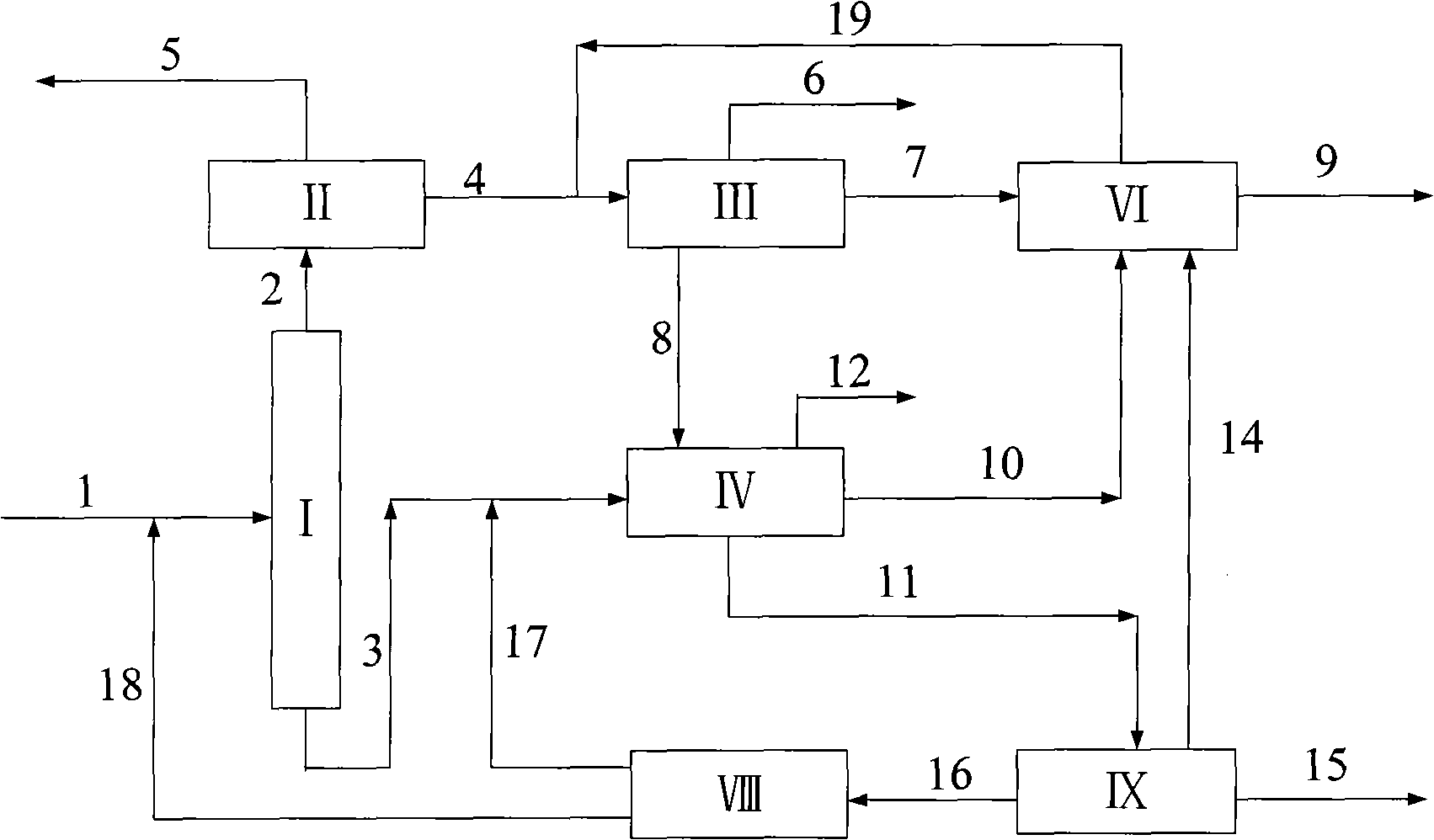
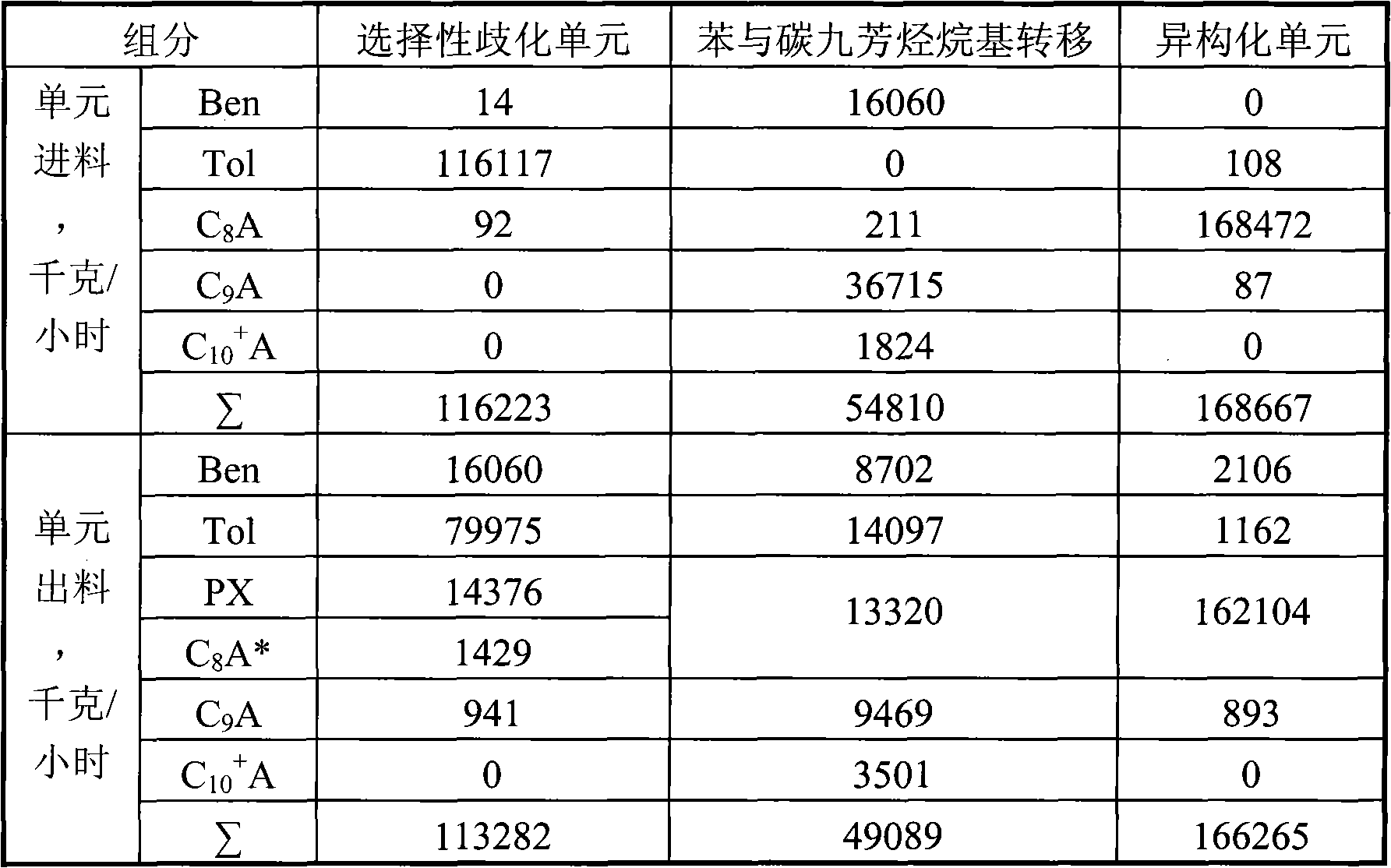
[0026] according to figure 2 The process shown is based on the C in typical reformed depentanized oil 6 A~C 10 + The composition of various hydrocarbon substances is used as the basic data to investigate the ability of the present invention to produce p-xylene and benzene and the processing scale of each unit. The composition distribution of aromatics sent out from a typical reformer and the flow rate of each component used in this example are shown in Table 1.
[0027] The benzene and carbon nine aromatics transalkylation process unit uses a fixed-bed reactor filled with a β-zeolite catalyst containing 0.05% bismuth. The reaction conditions are: reaction temperature 385 ° C, pressure 3.0 MPa, weight space velocity 2.0 Hour -1 , a hydrogen / hydrocarbon molar ratio of 3.0. Aromatic raw materials are mixed with hydrogen and then passed through the catalyst bed from top to bottom for C 9 + A dealkylation reaction to generate benzene, toluene and C 8 a.
[0028] The tolue...
Embodiment 2
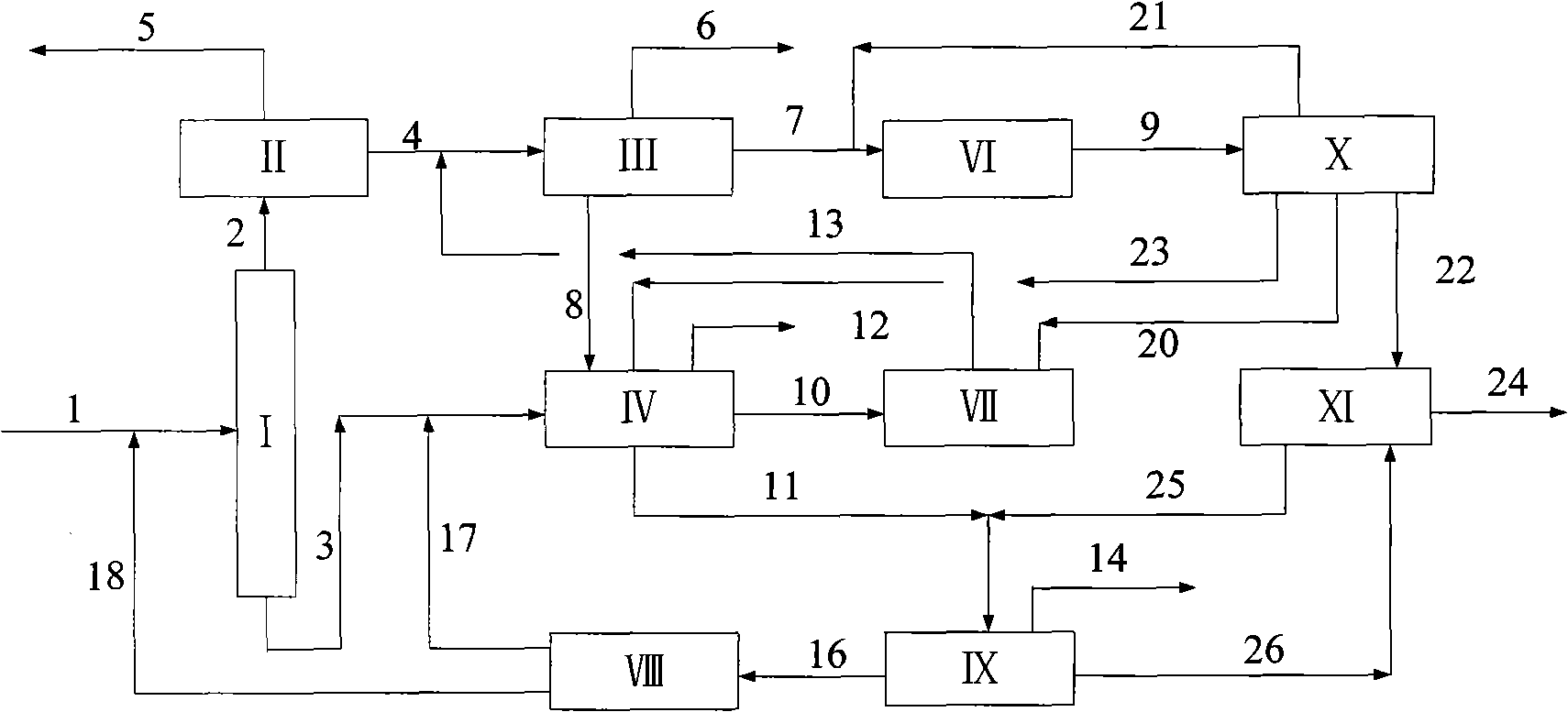
[0044] according to figure 2 The process shown is based on the C in typical reformed depentanized oil 6 A~C 10 + The composition of various hydrocarbon substances is used as the basic data to investigate the ability of the present invention to produce p-xylene and benzene and the processing scale of each unit. The composition distribution of aromatics sent out from a typical reformer and the flow rate of each component used in this example are shown in Table 1.
[0045] The benzene and carbon nine aromatics transalkylation process unit adopts a fixed-bed reactor, and the reactor is filled with a hydrogen-type MCM-22 zeolite catalyst containing 0.30% bismuth. The reaction conditions are: reaction temperature 460°C, pressure 41.0MPa, weight space velocity 3.0 hours -1 , a hydrogen / hydrocarbon molar ratio of 8.0. Aromatic raw materials are mixed with hydrogen and then passed through the catalyst bed from top to bottom for C 9 + A dealkylation reaction to generate benzene,...
Embodiment 3
[0056] according to figure 2 The process shown is based on the C in typical reformed depentanized oil 6 A~C 10 + The composition of various hydrocarbon substances is used as the basic data to investigate the ability of the present invention to produce p-xylene and benzene and the processing scale of each unit. The composition distribution of aromatics sent out from a typical reformer and the flow rate of each component used in this example are shown in Table 1.
[0057] The benzene and carbon nine aromatics transalkylation process unit uses a fixed-bed reactor filled with a hydrogen-type mordenite catalyst containing 0.10% bismuth. The reaction conditions are: reaction temperature 320°C, pressure 1.0MPa, weight space velocity 0.8 Hour -1 , a hydrogen / hydrocarbon molar ratio of 2.0. Aromatic raw materials are mixed with hydrogen and then passed through the catalyst bed from top to bottom for C 9 + A dealkylation reaction to generate benzene, toluene and C 8 a.
[0058...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com