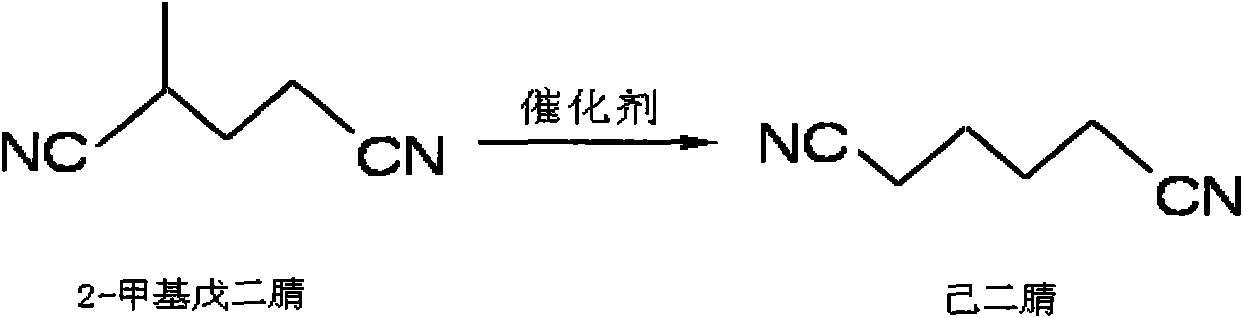
Method for preparing adiponitrile
A kind of technology of adiponitrile and butadiene nitrile, applied in the field of preparation of adiponitrile
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
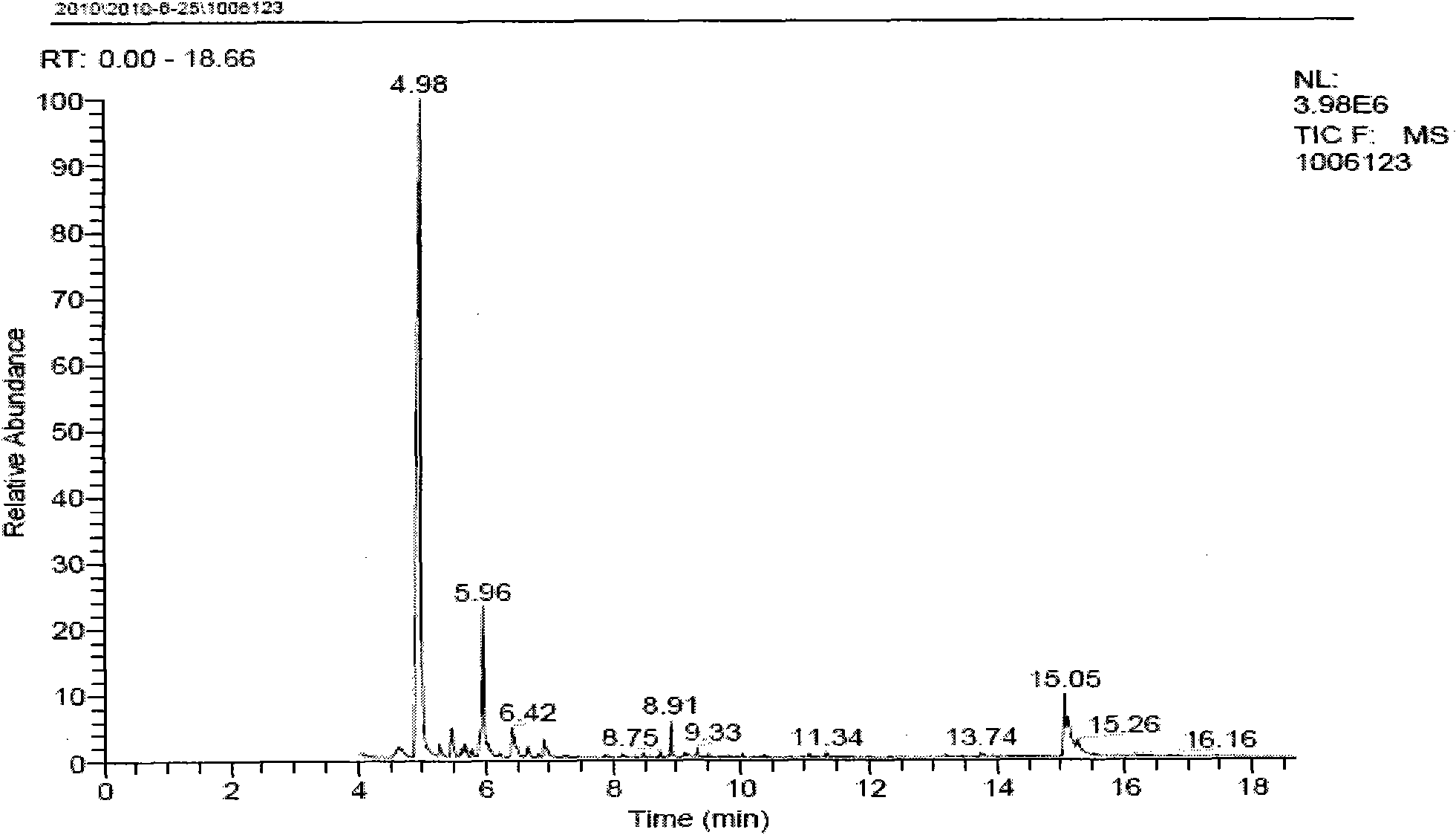
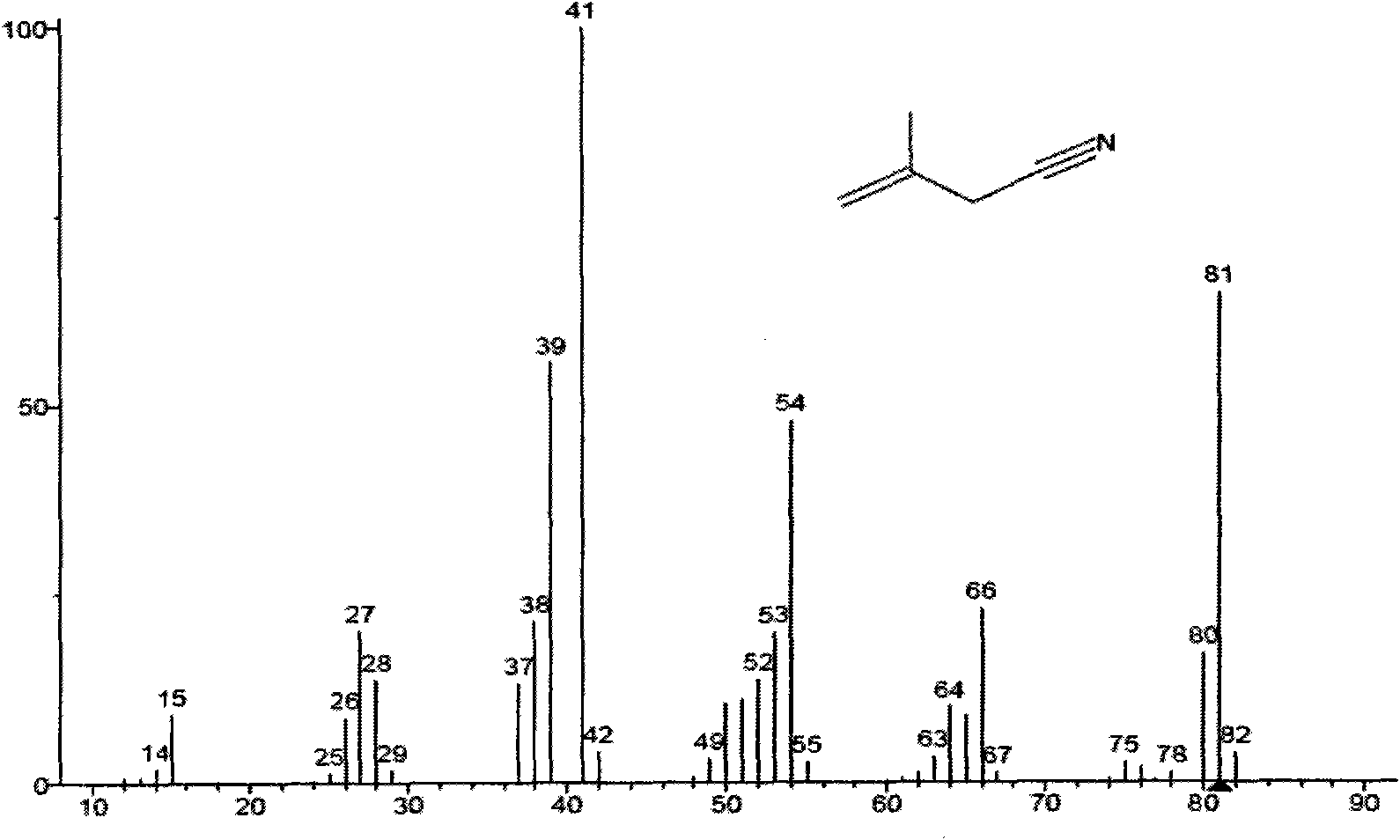
[0034] The method of the invention is illustrated by the following non-limiting representative examples. All parts, ratios and percentages are by weight unless otherwise indicated. The catalyst solution is composed of triphenylphosphine and Ni(COD) 2(“COD” refers to 1,5-cyclooctadiene) is prepared by mixing and dissolving in 3-pentenenitrile at a molar ratio of 4:1. Add 2-methylglutaronitrile to the catalyst solution, the molar ratio of 2-methylglutaronitrile to nickel is 100:1, and stir for 10 minutes to combine the catalyst solution and the substrate 2-methylglutaronitrile A homogeneous reaction mixture was formed. The reaction was continued for 4 hours at 100°C under the protection of argon, and the reaction was stopped. The reaction method is shown in the appendix figure 1 . The reaction mixture was analyzed using standard gas chromatography techniques, see attached Figure 2-6 . GC-MS model: Polaris-Q; manufacturer: Thermo Finnigan, USA. The conversion rate of 2-me...
Embodiment 2
[0036] The catalyst solution is composed of triphenylphosphine and Ni(COD) 2 It is prepared by mixing and dissolving in 3-pentenenitrile at a molar ratio of 4:1, and the reaction temperature is 170° C. Others are the same as in Example 1. The conversion rate of 2-methylglutaronitrile isomerization is 25%, and adiponitrile The selectivity is 82%.
Embodiment 3
[0038] The catalyst solution is composed of triphenylphosphine and Ni(COD) 2 It is prepared by mixing and dissolving in 3-pentenenitrile at a molar ratio of 4:1. The reaction temperature is 70° C., and the others are the same as in Example 1. The conversion rate of isomerization of 2-methylglutaronitrile is 0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com