Luminescence quenchers and fluorogenic probes for detection of reactive species
A compound and aromatic amine technology, applied in the field of manufacturing the aromatic amine compound, can solve problems such as changing protein structure, dimerization, protein fragmentation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0368] The synthetic scheme of embodiment 1-compound 1-4
[0369] Synthesis of compound 8
[0370]
[0371] To a solution of resorufin (2.13 g, 10 mmol) in 50 mL of anhydrous dimethylformamide was added sodium hydride (437 mg, 11 mmol, 60% dispersion in mineral oil) at 0°C. After stirring at 0°C for half an hour, N-phenylbis-trifluoromethanesulfonamide (4.3 g, 12 mmol) was added to the solution. The resulting mixture was stirred at room temperature overnight, then quenched with water. Then 1N hydrochloric acid was added to the mixture to acidify the solution to pH 2. Then ethyl acetate was added. The organic layer was separated and washed with brine, dried over anhydrous sodium sulfate and evaporated in vacuo. The residue was purified by silica gel column chromatography to give compound 8.
[0372] Synthetic compound 1
[0373]
[0374] Palladium(II) acetate (2 mg, 1% mmol), 2,2'-bis(diphenylphosphine)-1,1'-binaphthyl (BINAP) (9 mg, 1.5% mmol) and cesium carbonate...
Embodiment 2
[0380] The synthetic scheme of embodiment 2-compound 5-7
[0381] Synthesis of compound 9
[0382]
[0383] To a solution of naphthofluorescein (4.3 g, 10 mmol) in 50 mL of anhydrous dimethylformamide was added sodium hydride (437 mg, 11 mmol, 60% dispersion in mineral oil) at 0°C. After stirring at 0 °C for half an hour, methoxymethyl chloride (MOMCl) (0.76 mL, 10 mmol) was added to the solution. The resulting mixture was stirred at room temperature overnight, then quenched with water. Then 1N hydrochloric acid was added to the mixture to acidify the solution to pH 2. Then ethyl acetate was added. The organic layer was separated and washed with brine, dried over anhydrous sodium sulfate and evaporated in vacuo. The residue was purified by silica gel column chromatography to give compound 9.
[0384] Synthesis of compound 10
[0385]
[0386] To a solution of compound 9 (476 mg, 1 mmol) and pyridine (0.32 mL, 4 mmol) in anhydrous dichloromethane was added trifluoro...
Embodiment 3
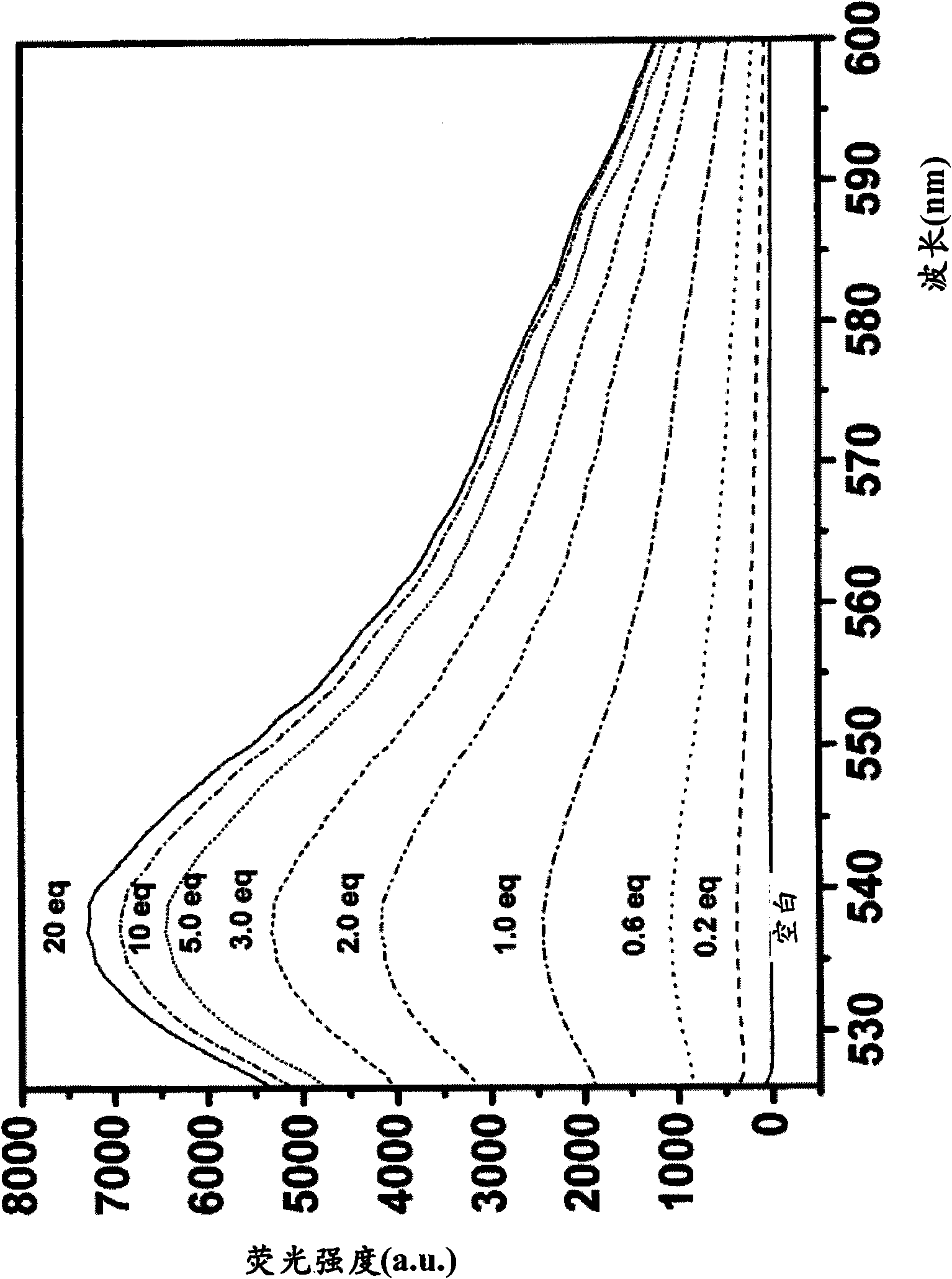
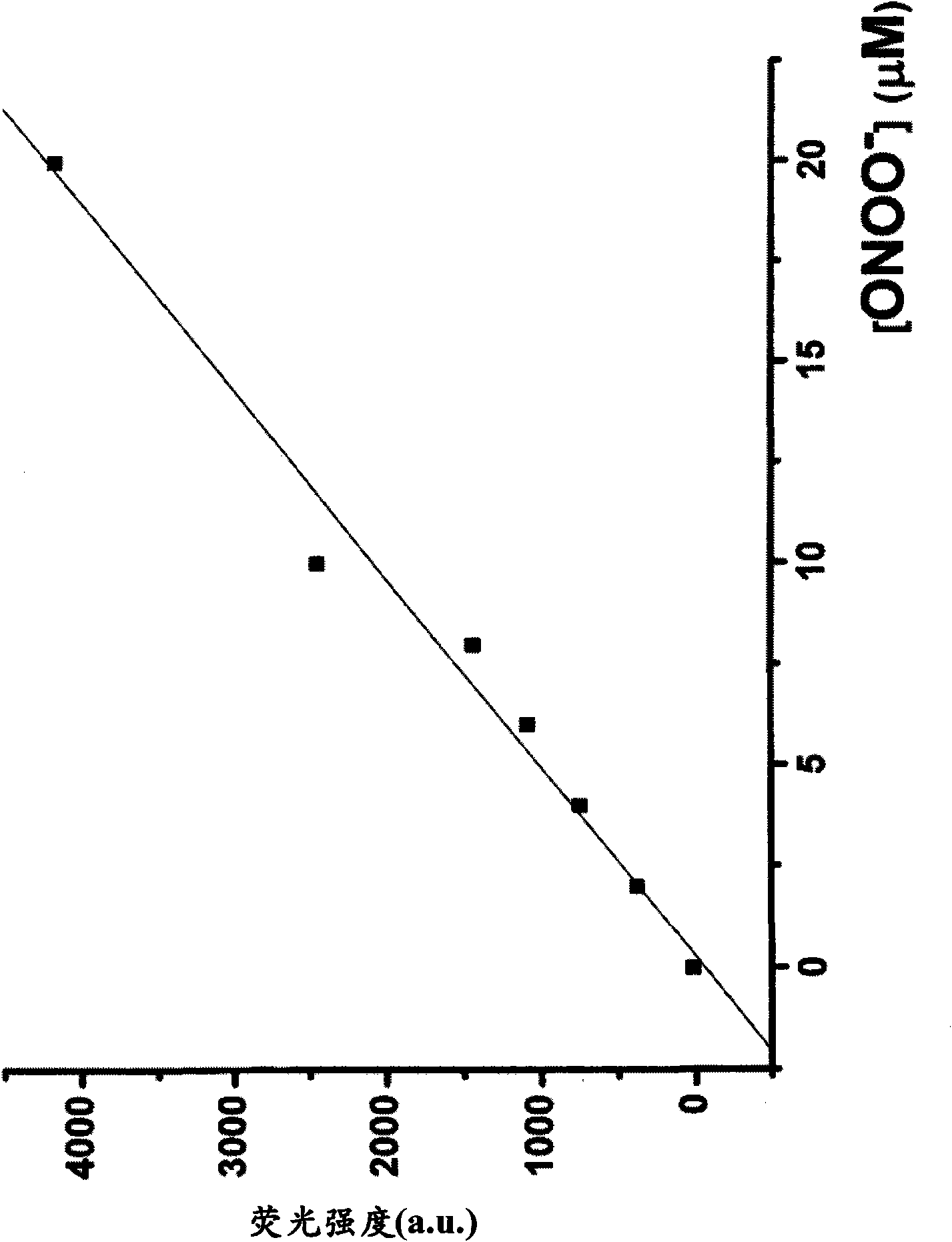
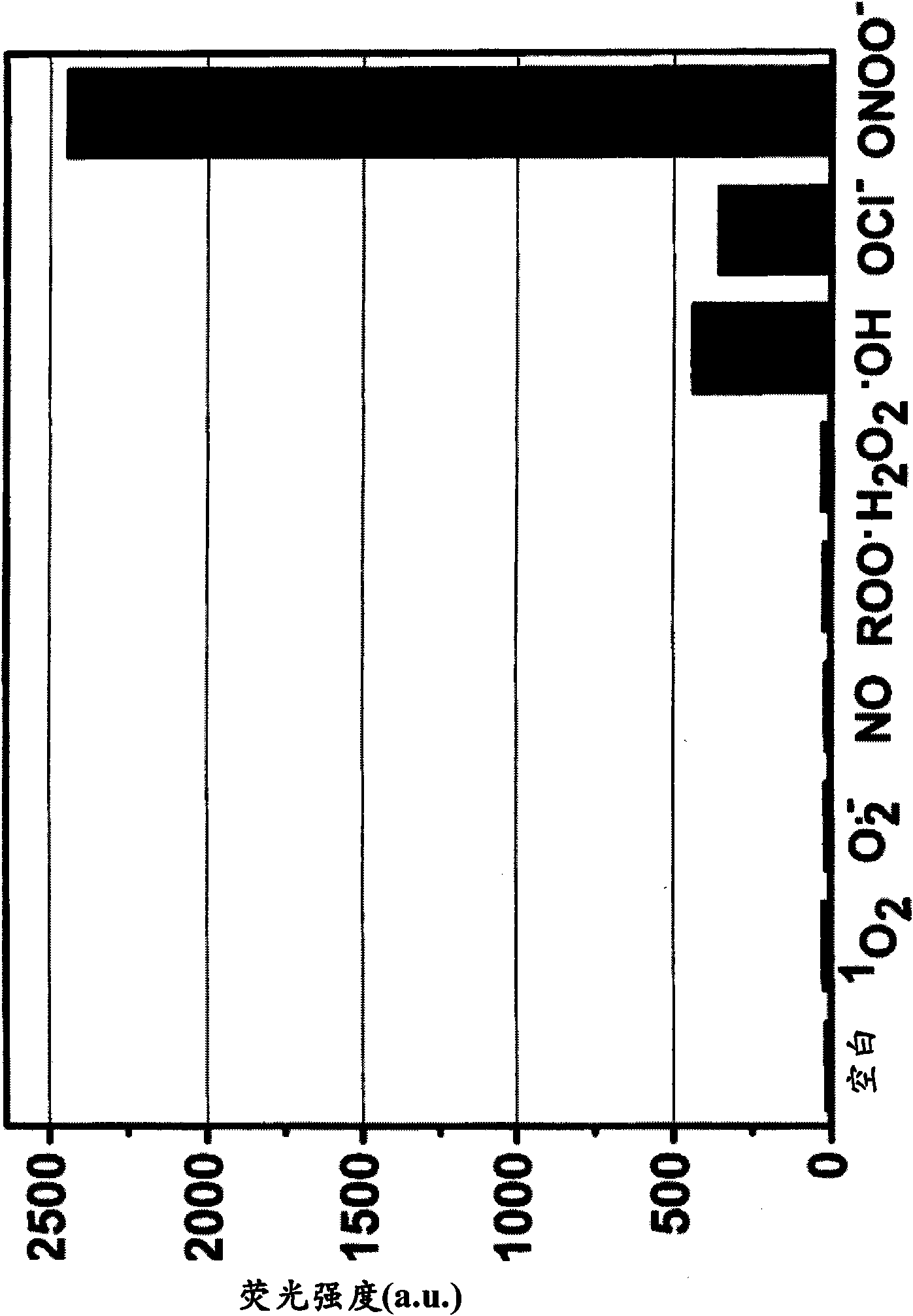
[0395] Example 3 - Evaluation of Fluorescence of Quenching Compounds
[0396] Compounds 1-7 obtained in Examples 1 and 2 were each dissolved in DMF to a concentration of 10 mM, and then the solution was diluted to 10 μM with 0.1 M phosphate buffer (pH 7.4). The fluorescence spectrum of the 10 μM compound solution was measured with a Hitachi F2500 fluorimeter, and the photomultiplier tube was set to 700V. The excitation and emission slit widths are both 2.5 nm. Measurements were performed at an excitation wavelength of 600 nm. The results showed that the absolute fluorescence intensities of compounds 1-7 were all less than 10. Therefore, none of compounds 1-7 are practically fluorescent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Extinction coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com