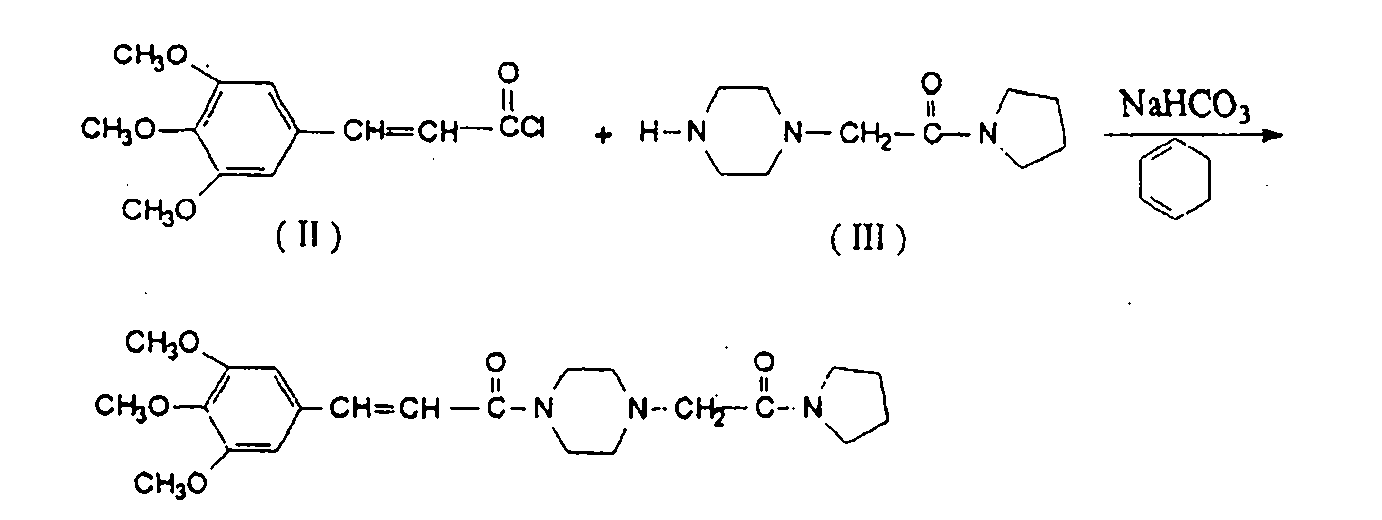
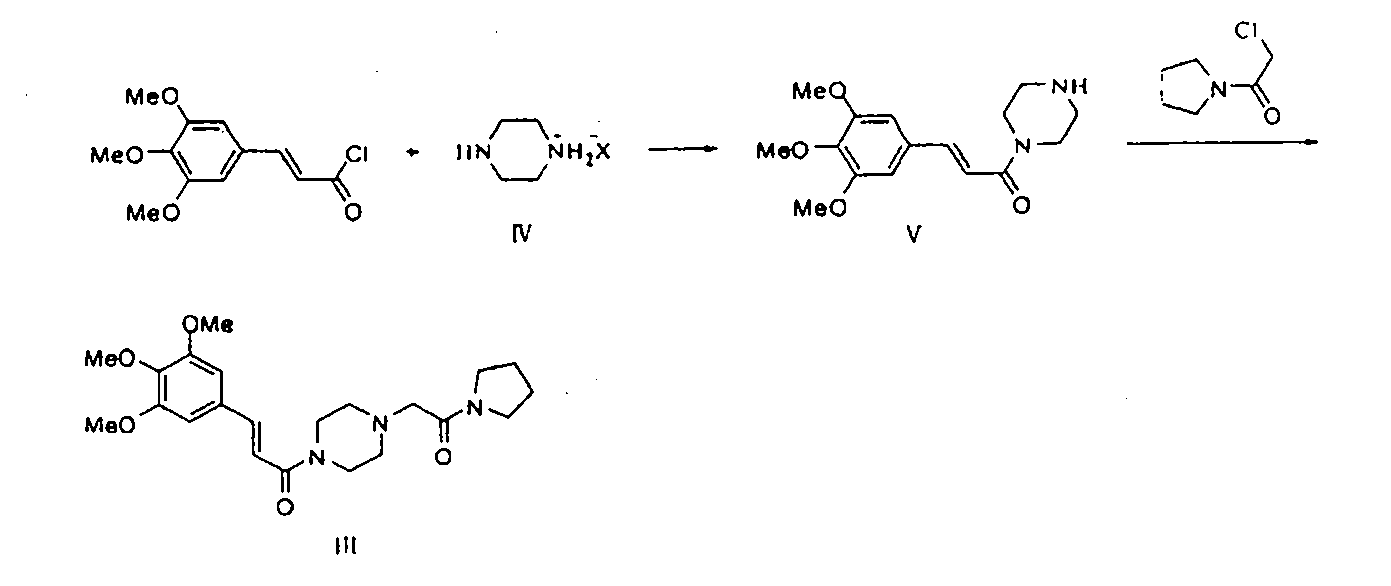
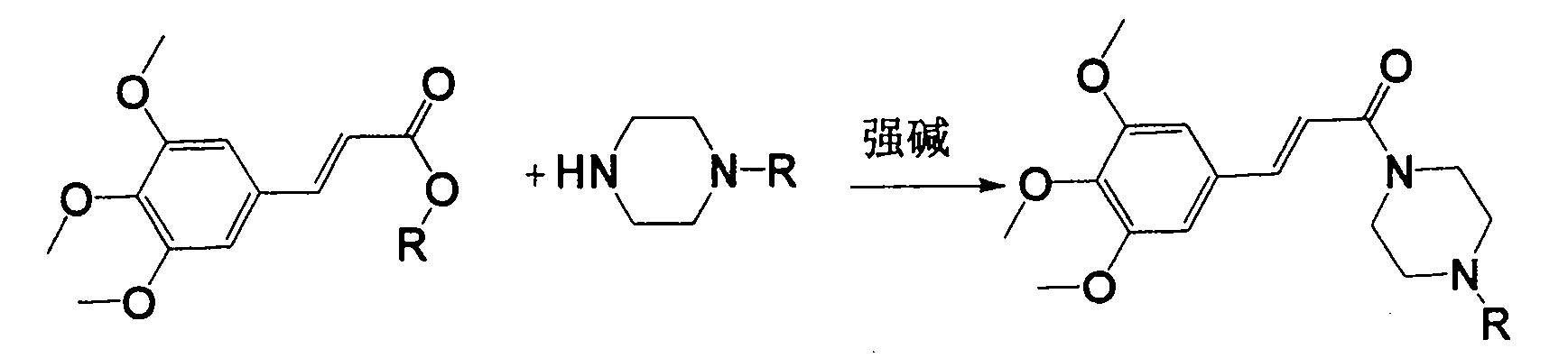
Method for preparing cinepazide free alkali
A technology of free base and piperazine, applied in the field of synthesis of cinepazide free base, can solve the problems of increasing investment in industrialized equipment, speeding up equipment depreciation, and high equipment requirements, and achieving stable product quality, enhanced nucleophilicity, environmental protection less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Mix 5.1g (0.02mol) of methyl 3,4,5-trimethoxycinnamate and 5.16g (0.06mol) of anhydrous piperazine in a 100ml single-necked bottle, add 5ml of dry N,N-dimethylformamide , stirred, and under a water bath, 2.2 g (0.06 mol) of NaH (65%) was added, and after adding, no bubbles were generated. Heat to reflux for 10 hours. Stop heating, and add 20ml of water dropwise under ice bath. Adjust the pH to 1-2 with 4M hydrochloric acid aqueous solution, and extract the aqueous phase with dichloromethane (20ml*3). Under the ice bath of the water phase, adjust the pH to about 12 with NaOH, extract with dichloromethane (25ml*3), combine the organic phases, extract with saturated aqueous sodium carbonate solution (15ml*2), and concentrate the organic phase to obtain 3.67g of light yellow oil, which is Cinepazide intermediate (60% yield).
Embodiment 2
[0020] Mix 5.1g (0.02mol) of methyl 3,4,5-trimethoxycinnamate and 7.89g 1-piperazineacetylpyrrolidine (0.04mol) in a 100ml single-necked bottle, add 5ml of dry N,N-di Methylformamide was stirred, and under a water bath, 2.2 g (0.06 mol) of NaH (65%) was added. After the addition, no bubbles were generated. Heat to reflux for 10 hours. Stop heating, and add 20ml of water dropwise under ice bath. 4M hydrochloric acid aqueous solution was used to adjust the pH to 1-2, and the aqueous phase was extracted with dichloromethane (20ml*3). Under the ice bath of the water phase, adjust the pH to about 12 with NaOH, extract with dichloromethane (25ml*3), combine the organic phases, extract with aqueous acetic acid (acetic acid: water = 15:100) (15ml*2), concentrate the organic phase to obtain shallow 4.58 g of reddish-brown oily substance is cinepazide free base (yield 55%).
Embodiment 3
[0022] Mix 8.2g (0.025mol) of benzyl 3,4,5-trimethoxycinnamate and 9.9g 1-piperazineacetylpyrrolidine (0.05mol) in a 100ml single-necked bottle, stir and add NaH (65%) 2.95g (0.08mol), slowly heated until the solid dissolved. Keep the temperature t=105~110°C for 8 hours, stop heating, add 20ml of water dropwise under ice bath. 4M hydrochloric acid aqueous solution was used to adjust the pH to 1-2, and the aqueous phase was extracted with dichloromethane (20ml*3). The aqueous phase was placed in an ice bath, adjusted to about PH=12 with sodium hydroxide, extracted with dichloromethane (25ml*3), combined with the organic phase, extracted with aqueous acetic acid (acetic acid: water = 15:100) (15ml*2), concentrated the organic phase Obtained 4.35 g of a light reddish-brown oily substance, which was cinepazide free base (yield 41%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com