Method for preparing high purity nano cobalt hydroxide
A cobalt hydroxide, nano-scale technology, applied in the direction of cobalt oxide/cobalt hydroxide, nanostructure manufacturing, nanotechnology, etc., can solve the problems of phase analysis, the existence of impurity, irregular particles, and impure product purity, etc., to achieve Uniform particle size distribution, excellent fluidity, and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
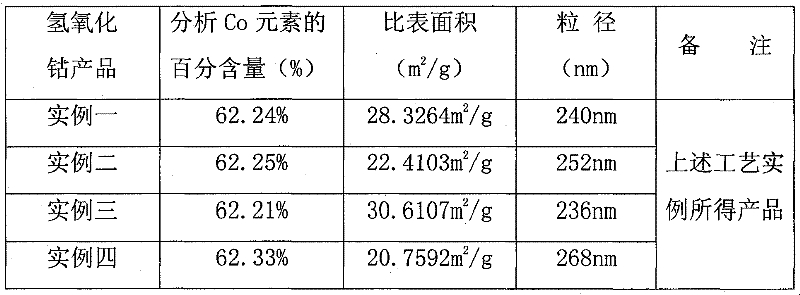
Embodiment 1
[0026] Pre-prepared solutions: a cobalt sulfate aqueous solution with a concentration of 0.2mol / l, an aqueous solution of sodium hydroxide with a concentration of 3.0mol / l, an aqueous solution of a cobalt ion complexing agent with a concentration of 5.0mol / l, and an aqueous solution of reagent A with a concentration of 2.0mol / l Polyacrylamide aqueous solution, appropriate amount of additive aqueous solution. Then, the prepared cobalt sulfate aqueous solution, sodium hydroxide aqueous solution, cobalt ion complexing agent aqueous solution, and polyacrylamide aqueous solution are successively input into the reactor with stirring according to their flow ratios of 1:0.68:0.048:0.058. , control the reaction temperature to 20°C. Among them, the cobalt hydroxide suspension can be obtained after the reaction between the cobalt sulfate aqueous solution and the sodium hydroxide aqueous solution is complete, and its chemical reaction formula is CoSO 4 +2NaOH=Co(OH) 2 ↓+Na 2 SO 4 The ...
Embodiment 2
[0028] Pre-prepared solution: the cobalt sulfate aqueous solution with a concentration of 1.0mol / l, the aqueous sodium hydroxide solution with a concentration of 5.0mol / l, the cobalt ion complexing agent aqueous solution with a concentration of 7.5mol / l, and the aqueous solution of reagent A with a concentration of 6.5mol / l l of hydrazine hydrate solution, an appropriate amount of additive solution. The prepared cobalt sulfate aqueous solution, sodium hydroxide aqueous solution, cobalt ion complexing agent aqueous solution, and hydrazine hydrate aqueous solution are respectively successively input into the reactor with stirring according to their flow ratios of 1:0.70:0.050:0.060, and the control The reaction temperature was 35°C. Among them, the cobalt hydroxide suspension can be obtained after the reaction between the cobalt sulfate aqueous solution and the sodium hydroxide aqueous solution is complete, and its chemical reaction formula is CoSO 4 +2NaOH=Co(OH) 2 ↓+Na 2 SO...
Embodiment 3
[0030] Pre-prepared solution: a cobalt sulfate aqueous solution with a concentration of 1.5mol / l, an aqueous sodium hydroxide solution with a concentration of 8.0mol / l, an aqueous solution of a cobalt ion complexing agent with a concentration of 10.0mol / l, and an aqueous solution of reagent A with a concentration of 11.0mol / l l sodium borohydride aqueous solution, appropriate amount of additive aqueous solution. The prepared cobalt sulfate aqueous solution, sodium hydroxide aqueous solution, cobalt ion complexing agent aqueous solution, and sodium borohydride aqueous solution are respectively successively input into the reactor with stirring according to their flow ratios of 1:0.72:0.052:0.062, The reaction temperature was controlled to be 50°C. Among them, the cobalt hydroxide suspension can be obtained after the reaction between the cobalt sulfate aqueous solution and the sodium hydroxide aqueous solution is complete, and its chemical reaction formula is CoSO 4 +2NaOH=Co(OH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com