Dihydroartemisinin beta-cyclodextrin inclusion compound, preparation method thereof and antimalarialdrug with same
A technology of cyclodextrin inclusion complex and dihydroartemisinin, which can be applied to medical preparations containing active ingredients, anti-infective drugs, drug combinations, etc. Problems such as public reports of dextrin inclusion, to achieve the effects of improving water-insoluble properties, improving storage conditions, and extending storage validity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of dihydroartemisinin β-cyclodextrin inclusion compound
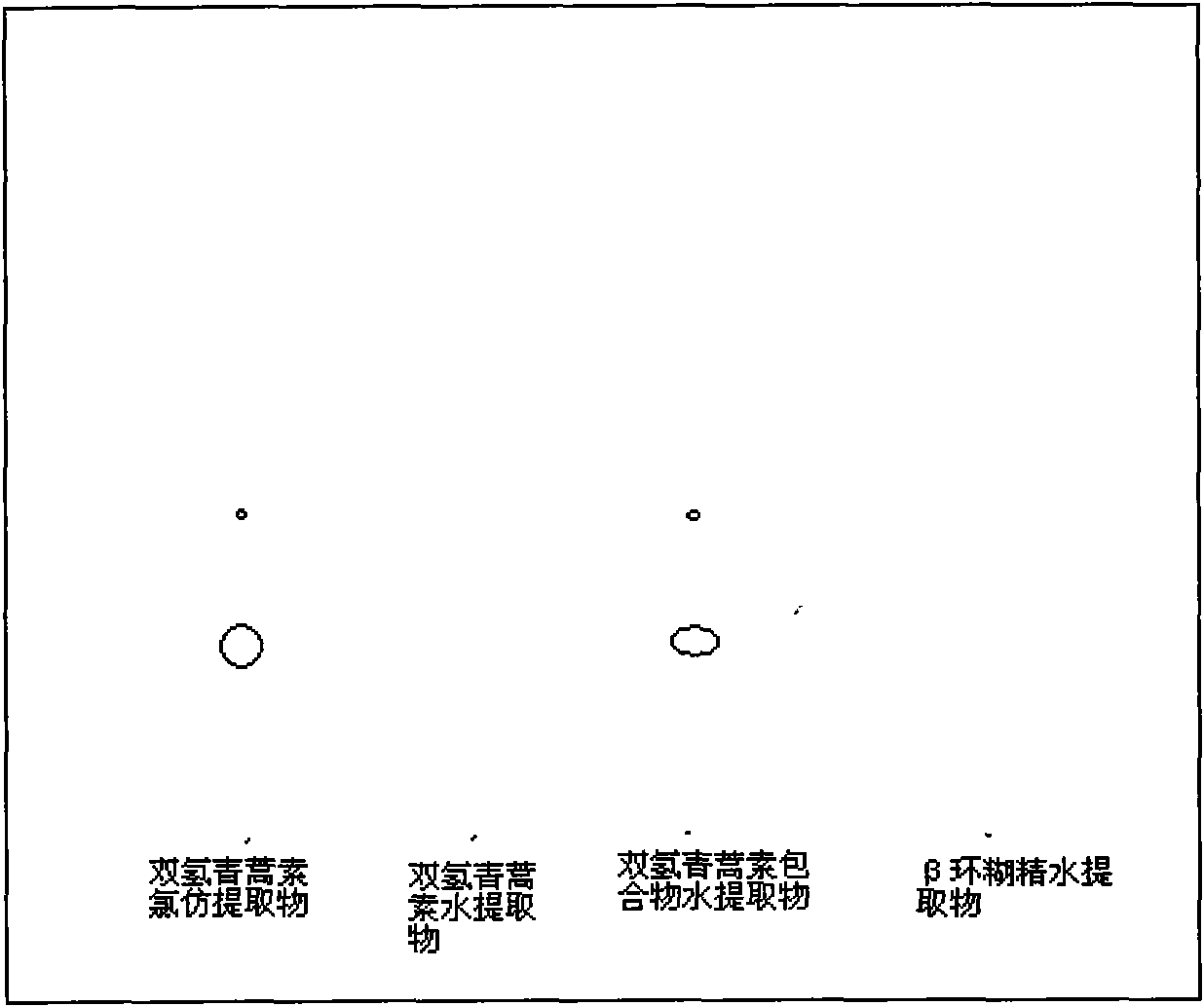
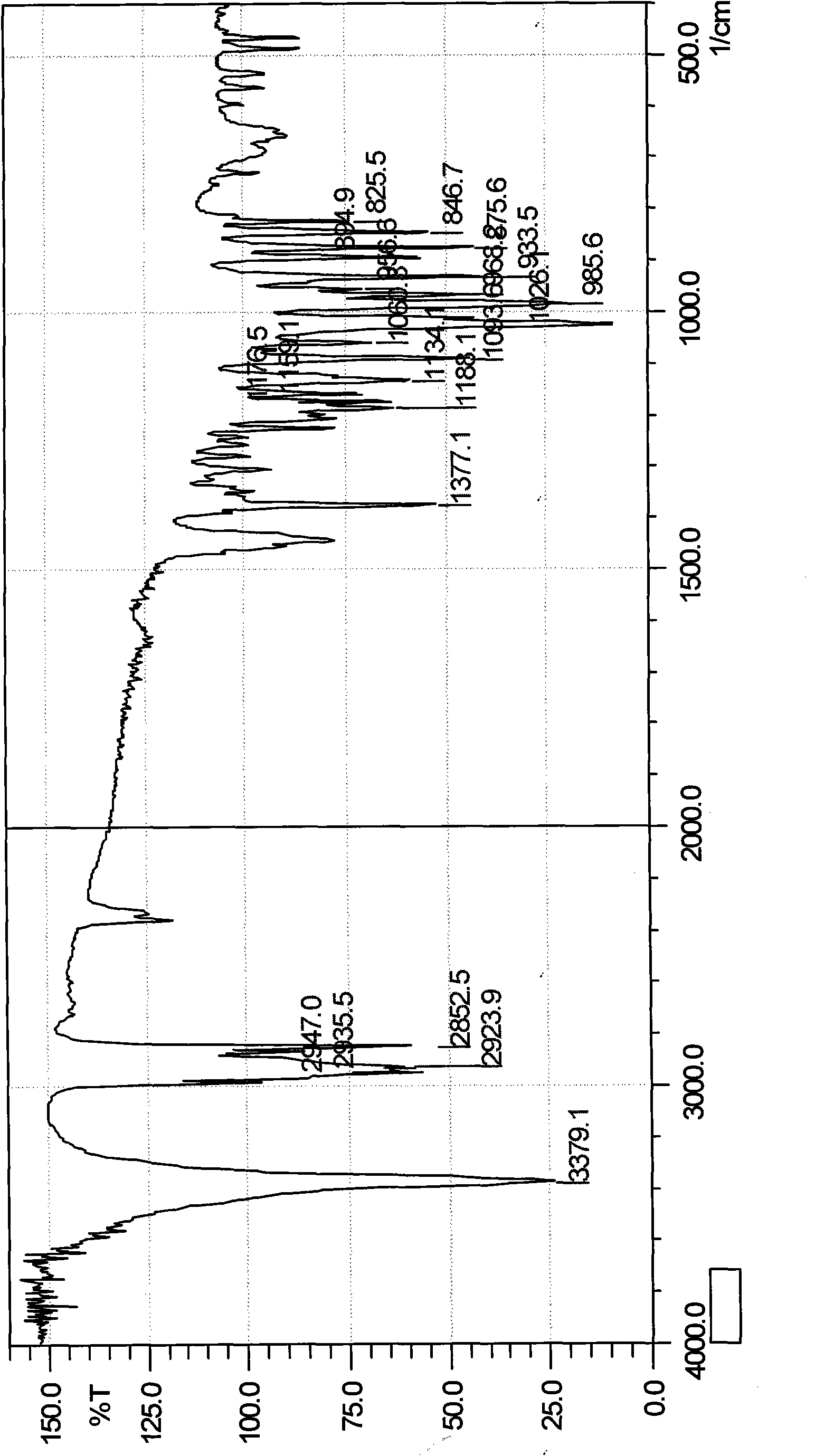
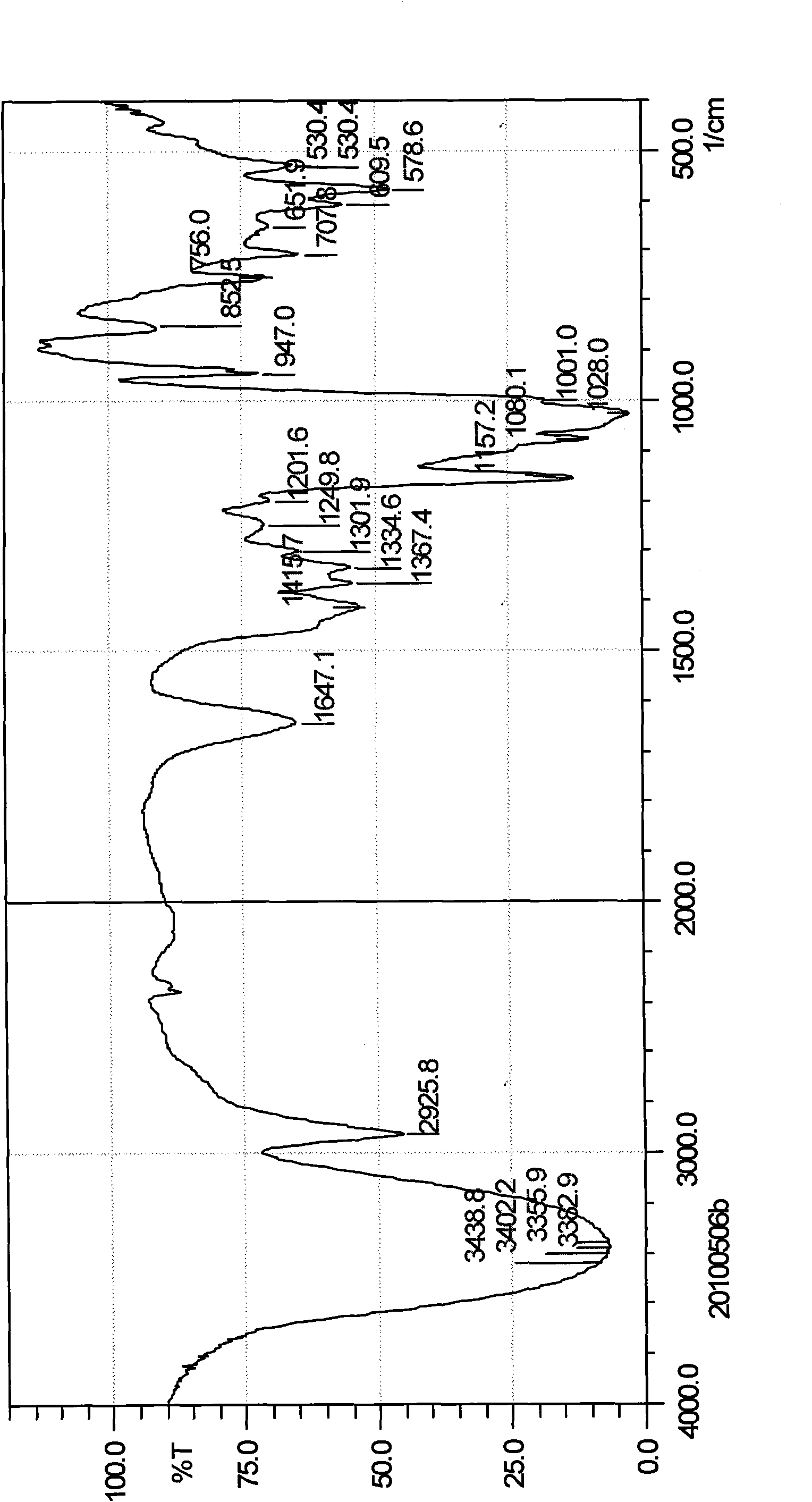
[0027] Dissolve 0.1g of dihydroartemisinin in 10ml of acetone to obtain a solution of dihydroartemisinin in acetone, set aside, then take 2g of β-cyclodextrin and dissolve it in 110ml of distilled water; Add dihydroartemisinin acetone solution to the aqueous solution, continue to stir for 4 hours, let it stand overnight, evaporate the acetone under reduced pressure, filter, and freeze-dry the filtrate to obtain 1.8 g of white powder, which is dihydroartemisinin β-cyclodextrin package Compound (referred to as dihydroartemisinin inclusion compound), detected by ultraviolet spectroscopy, the content of dihydroartemisinin in the obtained inclusion compound (the ratio of dihydroartemisinin in the inclusion compound) is 5.14 %. The calculated inclusion yield (actually measured content of dihydroartemisinin × yield of inclusion compound ÷ input amount of dihydroartemisinin) is: 92.52%.
[0028] ...
Embodiment 2
[0042] Example 2: Preparation of dihydroartemisinin β-cyclodextrin inclusion compound
[0043] Dissolve 1g of dihydroartemisinin in 120ml of acetone to obtain a solution of dihydroartemisinin in acetone, and then take 60g of β-cyclodextrin and dissolve it in 3333ml of distilled water; Add dihydroartemisinin acetone solution to the saturated aqueous solution of β-cyclodextrin, continue ultrasonication for 1 hour, let it stand overnight, evaporate the acetone under reduced pressure, filter, and freeze-dry the filtrate to obtain a white powder, which is confirmed by thin-layer chromatography to be dihydroartemisinin The hydrogen artemisinin β-cyclodextrin inclusion compound is detected by the ultraviolet spectrum detection method, and the content of dihydroartemisinin in the obtained inclusion compound is 2.01%, and the inclusion rate is 98.89%.
Embodiment 3
[0044] Example 3: Preparation of dihydroartemisinin β-cyclodextrin inclusion compound
[0045] Take 1g of dihydroartemisinin and dissolve it in 150ml of ethanol to obtain dihydroartemisinin ethanol solution, set aside, then take 10g of β-cyclodextrin and dissolve it in 833ml of distilled water; Add dihydroartemisinin in acetone solution, continue to stir for 2h, let it stand overnight, evaporate ethanol under reduced pressure, filter, and dry the filtrate under reduced pressure and low temperature to obtain 9.1g of white powder, which is dihydroartemisinin β-cyclodextrin The clathrate is detected by the ultraviolet spectroscopic detection method, and the content of dihydroartemisinin in the clathrate obtained is 7.53%, and the clathrate yield is 68.52%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com