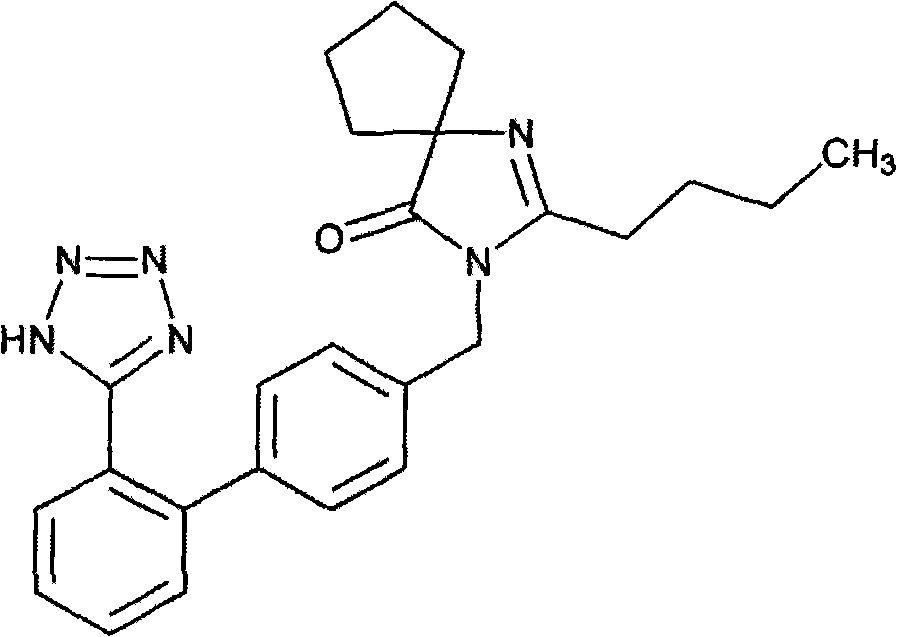
Medicinal composite containing irbesartan
A technology of irbesartan and composition, which is applied in the direction of drug combination, medical preparations containing active ingredients, and pharmaceutical formulations, and can solve problems affecting the stability of pharmaceutical preparations, the impact of dissolution of pharmaceutical preparations, and the impact on product stability, etc. Achieve the effects of reducing production costs, simplifying prescriptions, and reducing potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
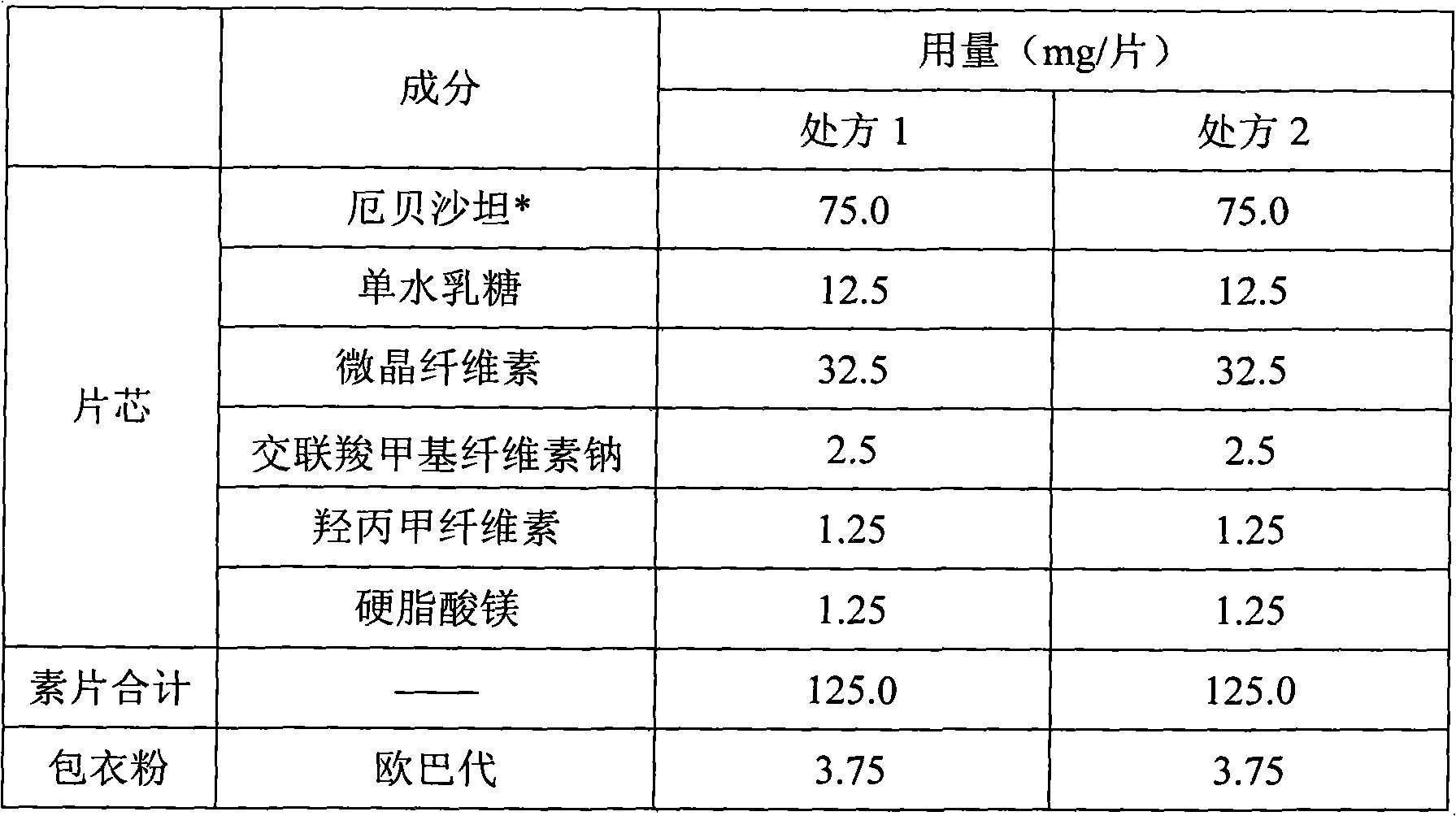
[0036] Embodiment 1 Comparison experiment of different particle sizes of irbesartan:
[0037]
[0038] * Irbesartan adopts two particle sizes, the particle size of irbesartan used in prescription 1 is D(V, 0.9)=150 μm, and the particle size of irbesartan used in prescription 2 is D(V, 0.9)=312 μm.
[0039] Preparation process: Dissolve the prescribed amount of hypromellose in an appropriate amount of purified water to make an adhesive for later use; put irbesartan, lactose monohydrate, microcrystalline cellulose, and croscarmellose sodium into Mix in the granulation pot to obtain mixture A; add the reserved binder to the mixture A in the granulation pot to granulate to obtain wet granules, granulate the wet granules and dry to obtain dry granules B, and then add the prescribed amount of stearin Magnesium acid was added to dry granule B, and mixed evenly to obtain mixture C, and the angle of repose of mixture C was measured. Finally, mixture C was compressed into tablets o...
Embodiment 2
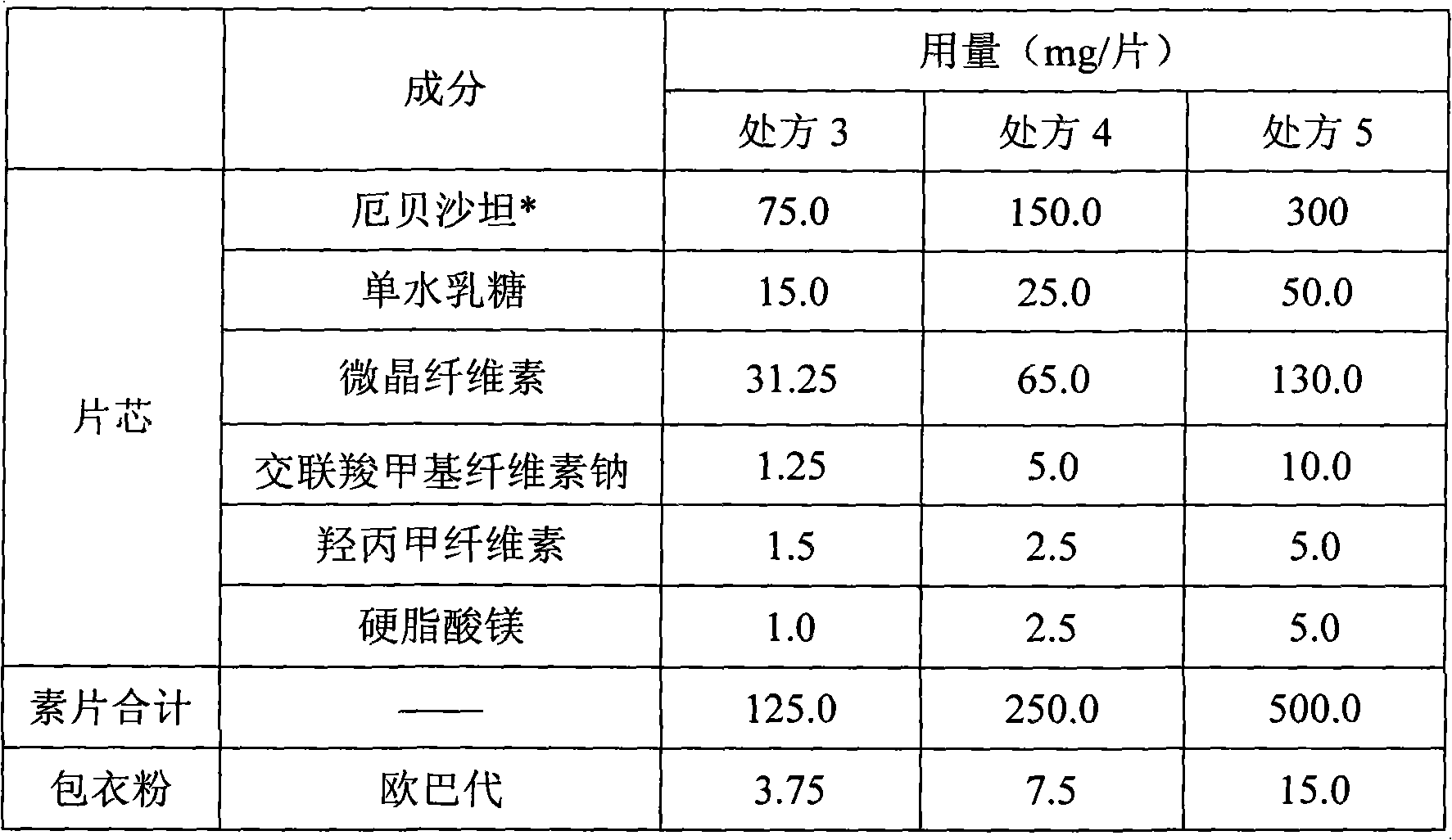
[0041] Embodiment 2 unilateral irbesartan tablet:
[0042]
[0043] * The particle sizes of irbesartan in prescriptions 3-5 were D(V, 90)=37 μm, D(V, 90)=200 μm, D(V, 90)=84 μm, respectively.
[0044] Preparation Process:
[0045] 1. Dissolve the prescribed amount of hypromellose in an appropriate amount of purified water to make an adhesive;
[0046] 2. Put the prescribed amount of irbesartan, lactose monohydrate, microcrystalline cellulose, and croscarmellose sodium into a granulation pot and mix to obtain a mixture A;
[0047] 3. Add the binder in step 1 to the mixture A in the granulation pot to granulate to obtain wet granules B;
[0048] 4. Sizing the wet granules and drying them to obtain dry granules C;
[0049] 5. Add the prescribed amount of magnesium stearate into the dry granule C, and mix well to obtain the dry granule D;
[0050] 6. Compressing the dry granule D to obtain a tablet with a corresponding tablet weight;
[0051] 7. Use a high-efficiency coat...
Embodiment 3
[0052] Embodiment 3 compound irbesartan tablets:
[0053]
[0054]
[0055] * The particle sizes of irbesartan in prescriptions 6-8 are D(V,90)=50 μm, D(V,90)=84 μm, D(V,90)=84 μm, respectively.
[0056] Preparation Process:
[0057] 1. Dissolve the prescribed amount of hypromellose in an appropriate amount of purified water to make an adhesive;
[0058] 2. Put the prescribed amount of irbesartan, hydrochlorothiazide, lactose monohydrate, microcrystalline cellulose, and croscarmellose sodium into a granulation pot and mix to obtain mixture A;
[0059] 3. Add the binder in step 1 to the mixture A in the granulation pot to granulate to obtain wet granules B;
[0060] 4. Sizing wet granule B and then drying to obtain dry granule C;
[0061] 5. Add the prescribed amount of magnesium stearate into the dry granule C, and mix well to obtain the dry granule D;
[0062] 6. Compressing the dry granule D to obtain a tablet with a corresponding tablet weight;
[0063] 7. A hig...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com