Method for purifying copper electrolyte with minimal chemical reacting dose
A technology of copper electrolyte and electrolyte, which is applied in electrolysis process, electrolysis components, cells, etc., to achieve the effects of high power consumption, high precision control of process parameters, and improved arsenic removal efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The blister copper plate obtains pure copper by electrolysis. During the electrolysis process, high-purity copper is deposited as copper transfers from the anode to the cathode on the electrode. Since blister copper contains arsenic, nickel and other impurity metals, these impurities remain in the electrolyte during the electrolysis process. With the accumulation of impurities, it will cause high power consumption, decrease in chemical composition and quality, and a large amount of chemical reaction. Based on this state, the present invention adopts the following methods and steps to purify the copper electrolyte with the minimum amount of chemical reaction.
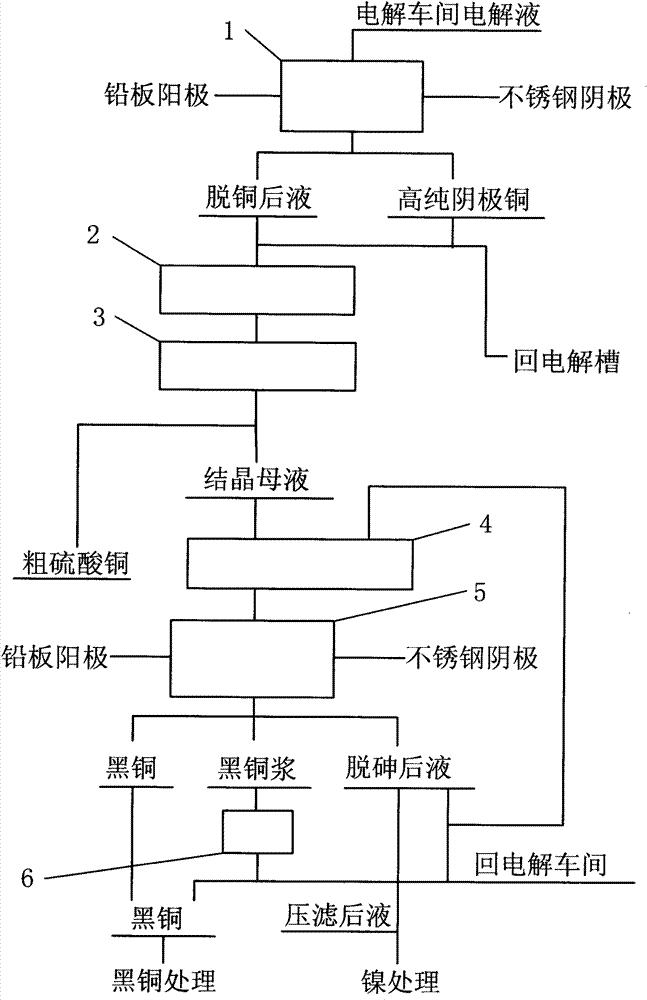
[0020] Below to figure 1 The process flow shown is described in detail
[0021] 1. Electrowinning copper removal step 1: Inject the copper electrolyte from the electrolytic workshop into the copper removal electrolytic cell, use the lead plate as the anode and the stainless steel plate as the cathode, and pass di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com