Method for directly synthesizing aniline from benzene and ammonia by one step
A benzene one-step, direct technology, applied in chemical instruments and methods, preparation of amino-substituted hydrogen atoms, molecular sieve catalysts, etc., can solve the problems of complex catalyst preparation process and unsatisfactory yield, and achieve short catalytic reaction time and simple preparation method. , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 weighs 0.1143g NiSO 4 , 0.1007g CuSO 4 ·5H 2 O, 0.0795g CeN 5 o 9 ·6H 2 O, 0.1004g VOSO 4 ·nH 2 O, 0.5127g TiCl 3 ·nH 2 O (35%) was dissolved in 2 mL of deionized water, heated and stirred until completely dissolved, and set aside. Weigh 5 parts of 1g TS-1 powder and place them in 5 branch test tubes respectively, and vacuumize at 25°C for 0.5 hours, the vacuum degree is 0.9×10 2 KPa, while maintaining the vacuum, pour 5 parts of metal salt solutions that have been prepared into the carriers in 5 branched test tubes while hot, and leave to age for 24 hours; °C until completely dry, then move the dried catalyst into a crucible and bake at 500 °C for 4 hours. Catalysts are marked as Ni / TS-1, Cu / TS-1, Ce / TS-1, V / TS-1, Ti / TS-1, and the weight percentage of the metal loading of the above five catalysts is 2.5%, and they are stored dry for later use .
Embodiment 2
[0019] Embodiment 2 weighed 0.2286g, 0.3429g, 0.4572g NiSO 4 According to the method of Example 1, Ni / TS-1 catalysts were prepared, wherein the nickel loading mass percentages were 5.0%, 7.5%, and 10.0%, respectively.
Embodiment 3
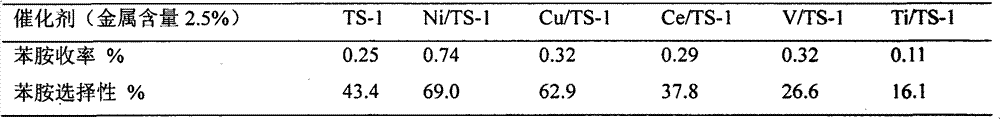
[0020] Example 3 Weigh TS-1, Ni / TS-1, V / TS-1, Cu / TS-1, Ce / TS-1, Ti / TS-1 respectively (wherein the metal loading mass percent is 2.5%) 0.3g into six 50mL two-necked bottles, add 5mL of benzene and 10mL of 25% ammonia water, stir and heat to 70°C, add 2mL of 30% H 2 o 2 , after which 2 mL of 30% H was added every 24 min 2 o 2 , adding a total of 10 mL of 30% H 2 o 2 , the reaction time was 2 hours, and the effects of different metal-loaded catalysts on the aniline yield and aniline selectivity were obtained. The results are shown in Table 1.
[0021] Table 1 Effect of different metal-loaded catalysts on the yield and selectivity of aniline
[0022]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com