New usage of formononetin-3'-sodium sulfonate
A technology of formononetin and sodium sulfonate, applied in medical preparations containing active ingredients, cardiovascular system diseases, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
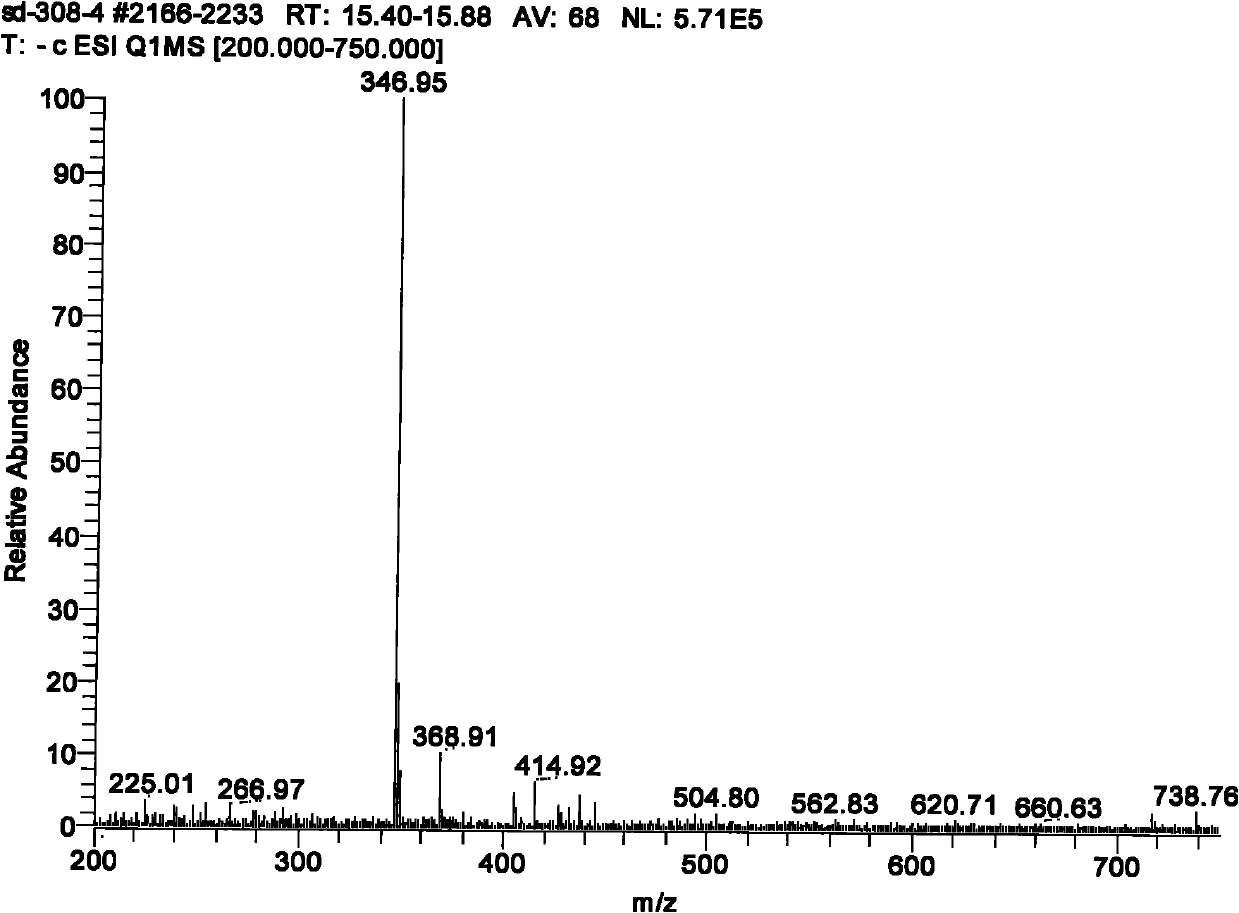
[0023] Preparation Example 1 Preparation of formononetin-3'-sodium sulfonate
[0024] Add 3 kg of formononetin (purchased from Xi'an Yangling Dongke Madison Pharmaceutical Company, batch number 081211, content 98.5%) into the reaction kettle, stir with a mixer and gradually add solvent 80% sulfuric acid until formononetin is fully dissolved, and then Add sulfonating agent 98% concentrated sulfuric acid, and the molar ratio of formononetin to sulfonating agent is 1:5. After stirring with a mixer, use a temperature adjusting device to make the temperature of the reaction solution 60°C and react for 6 hours to obtain formononetia The mixture of Su-3′-sulfonic acid and unreacted raw materials is cooled to room temperature, and a 10% sodium chloride aqueous solution of 5 times the total volume of the reactants is added, fully stirred, and left to stand to precipitate a white precipitate, which is filtered to obtain formononetin -3′-Sodium Sulfonate Crude Product; Use the recrystalliza...
preparation example 2
[0025] Preparation Example 2 Preparation of Formononetin-3'-Sodium Sulfonate Injection
[0026] Prescription: Formononetin-3'-sodium sulfonate 5.0g
[0028] Water for injection 1000ml
[0029]
[0030] Make 1000ml
[0031] Preparation method: Take formononetin-3'-sodium sulfonate 5.0g and sodium chloride 8.9g according to the prescription, dissolve with 1000ml water for injection, stir; add 50g activated carbon, stir for 20 minutes, and filter the solution through a microporous membrane Clear, packed in 25ml vials, 10ml per bottle, sterilized, and packed after inspection. Other inspection items should meet the requirements of the 2005 edition of the Chinese Pharmacopoeia for injection items.
preparation example 3
[0032] Preparation Example 3 Preparation of formononetin-3'-sodium sulfonate freeze-dried powder injection
[0033] Take 50g of formononetin-3'-sulfonate, add 2000ml of water for injection after mixing, add 8g of mannitol, stir to dissolve, ultrafiltration to obtain a pyrogenic clear liquid, pour it into a 10ml vial, 2ml / piece, According to freeze-dried powder injection process, freeze-dried powder injections are made into freeze-dried powder injections each containing 50 mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com