Preparation and application of 1,2,3-3H pyridine-heterocyclic compound
A compound and technology of six-membered heterocyclic rings, applied in the field of preparation and application of 1,2,3-3H pyridine heterocyclic compounds, can solve problems such as limited applications
Inactive Publication Date: 2010-11-03
EAST CHINA UNIV OF SCI & TECH
View PDF12 Cites 9 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, due to the resistance problem of imidacloprid and the cross-resistance between neonicotinoid insecticides due to structural similarity, the application of this type of compound is limited to a certain extent.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
preparation example Construction
Embodiment 1
Embodiment 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
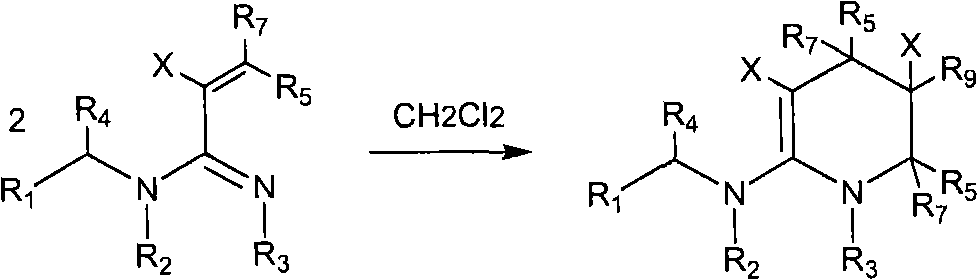
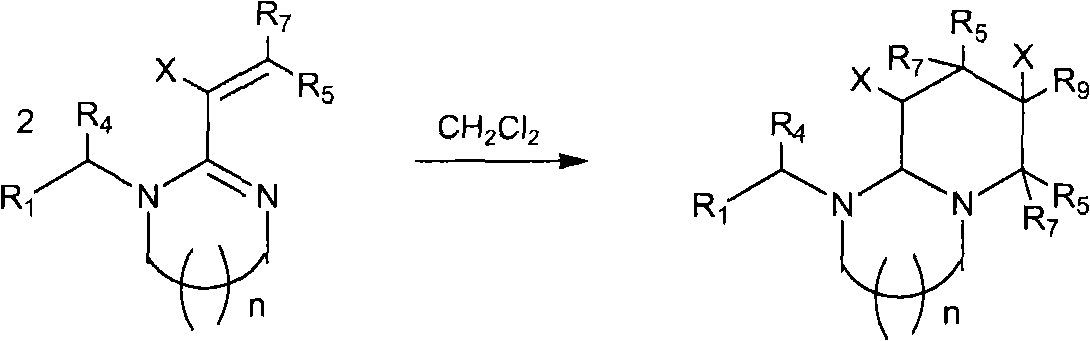
The invention relates to preparation and application of a 1,2,3-3H pyridine-heterocyclic compound. Specifically, an N heterocycle-contained or open ring-contained compound as shown in a general formula (A) in the specification, or an optical isomer and a cis-trans isomer thereof, or agriculturally-pharmaceutically acceptable salts are provided. The invention also relates to an agricultural composition containing the compound, or the optical isomer and the cis-trans isomer thereof or agriculturally-pharmaceutically acceptable salts, and the application of the agricultural composition. The compound and the derivatives thereof have extremely high insecticidal activity on agriculture and forestry pests, sanitary pests and pests harming animal health. The general formula (A) is shown in the specification.
Description
technical field The invention relates to a new neonicotinoid-like 1,2,3-3H pyridine heterocyclic insecticide, its preparation method and application. technical background In the past thirty years, the discovery of neonicotinoid insecticides is a milestone innovation in the field of insecticide research. In the mid-1980s, Bayer developed imidacloprid, the first neonicotinoid insecticide, and became one of the most successful new insecticides. Neonicotinoid insecticides represented by imidacloprid have high insecticidal activity, wide insecticidal spectrum, low toxicity to mammals and aquatic animals, good system properties, appropriate field stability and environmental friendliness, and become An important hot spot for the creation of new pesticides. Later, a series of neonicotinoid insecticides such as thiacloprid, clothianidin, thiamethoxam, acetamiprid, nitenpyram, and dinotefuran were developed successively (European patents 247477, 296453, 685477, 235725, 235725 , 315...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D405/14C07D401/14C07D471/04A01N43/40A01N43/90A01P7/00A01P7/04
CPCC07D401/14C07D471/04C07D405/14A01N43/78A01N43/90A01N43/40C07D487/04C07D409/14A61P33/00
Inventor 李忠钱旭红张文文徐晓勇邵旭升陶黎明宋恭华
Owner EAST CHINA UNIV OF SCI & TECH
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com