Method for purifying cefotetan acid crude products
A technology of cefotetan acid and purification method, which is applied in the field of preparation and purification of cephalosporin intermediates, can solve the problems of no isomer removal method, long time consumption, restriction of industrial production of cefotetan raw materials, etc., so as to shorten the transformation time The effect of reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
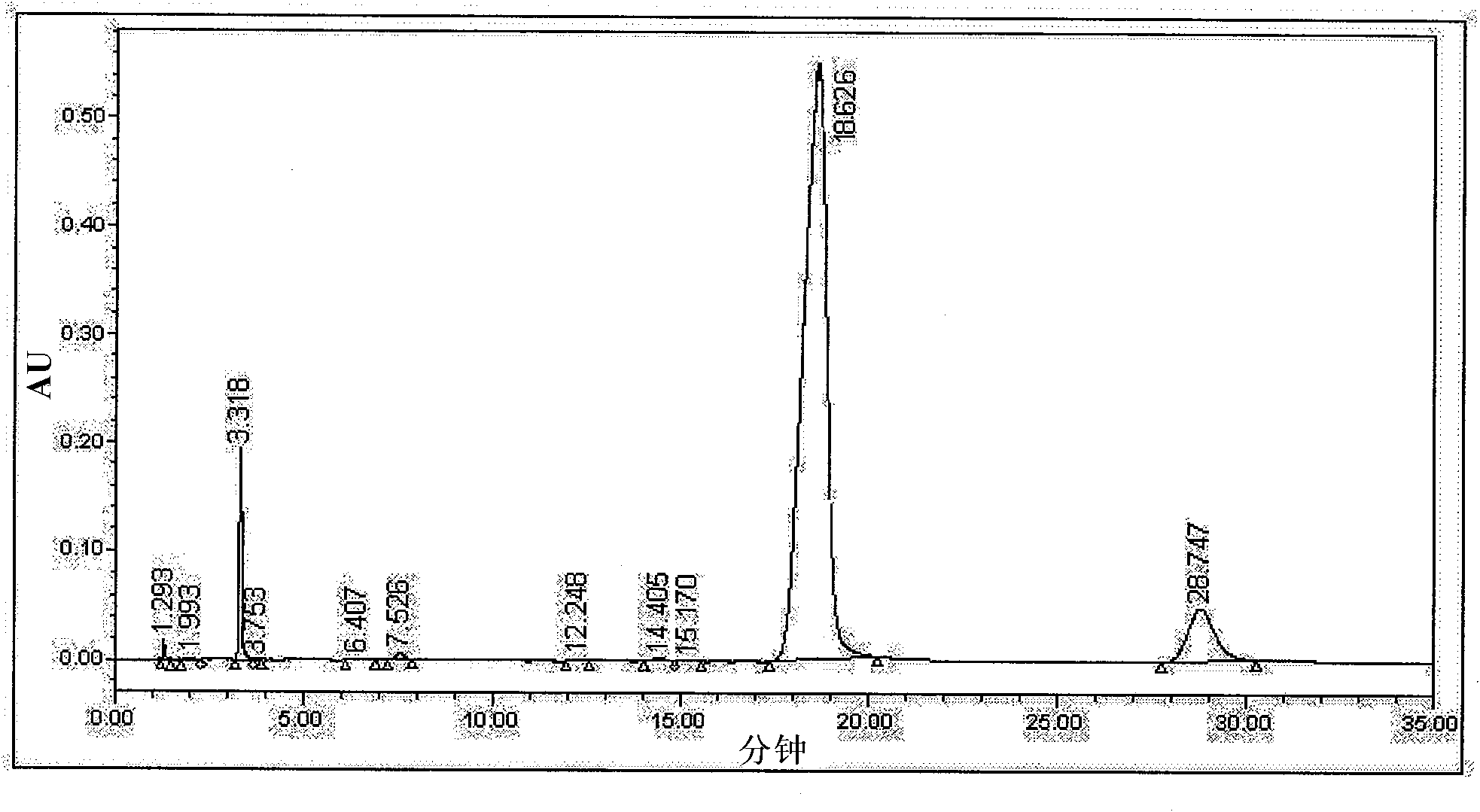
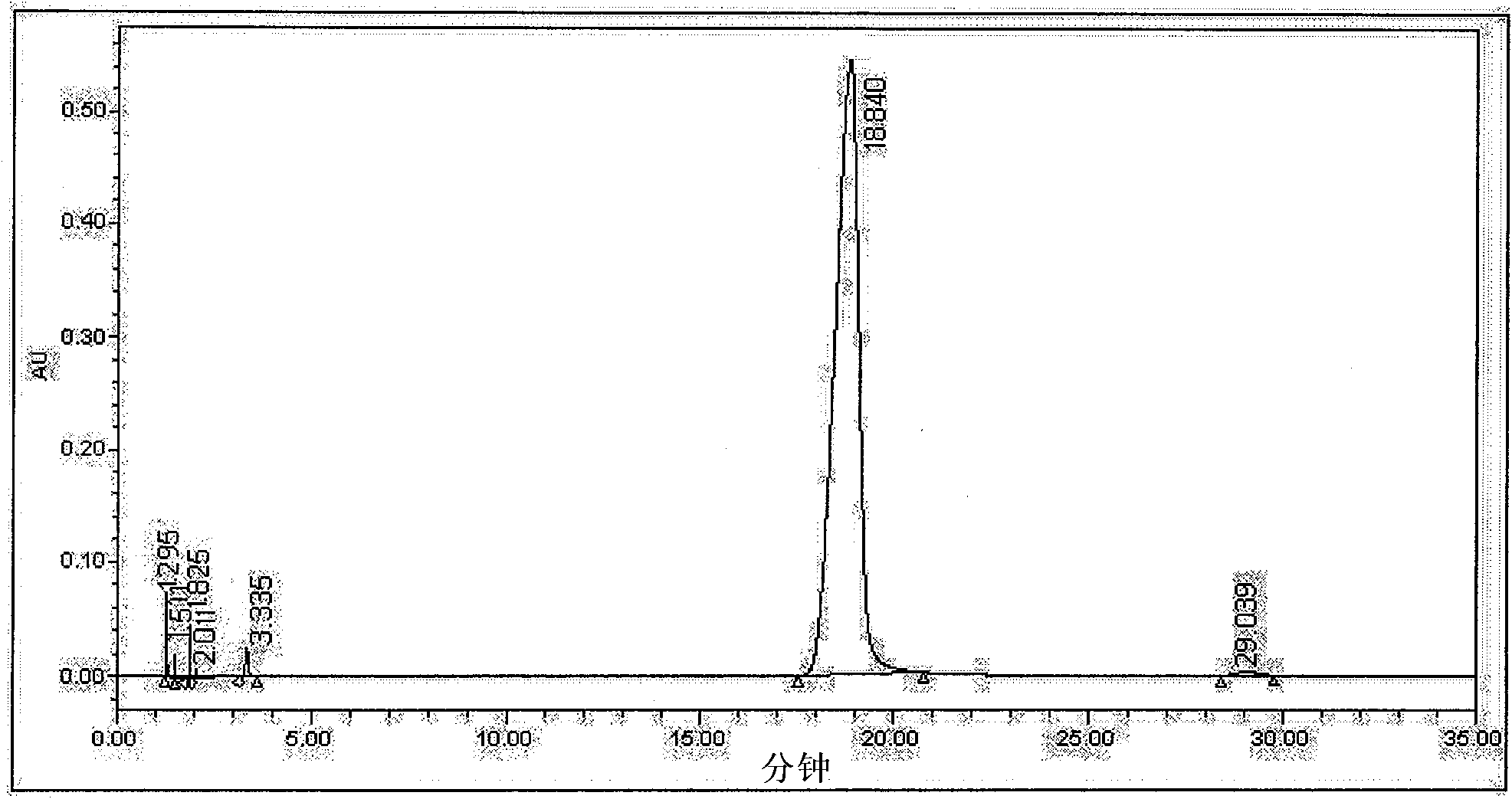
[0022] Get the cefotetan acid crude product 20g (HPLC detection isomer content is 8.95% for washing purification, see figure 1 ), was added to a 250ml reaction flask, and then 80ml of ethanol was added, stirred, suspended and washed for 30min at 15°C, vacuum filtered, and the filter cake was detected by HPLC, and the isomer content was 8.41%. Add 80 ml of cyclohexane to the filter cake in the first step, stir and wash at the same temperature for 30 minutes, vacuum filter, and detect by HPLC of the filter cake, the isomer content is 3.26%. Then add 120ml methyl alcohol to the filter cake of the second step, stir and wash 30min under the same temperature, vacuum suction filtration, filter cake HPLC detection, isomer content is 0.62%, see figure 2 . The overall yield is about 48%.
Embodiment 2
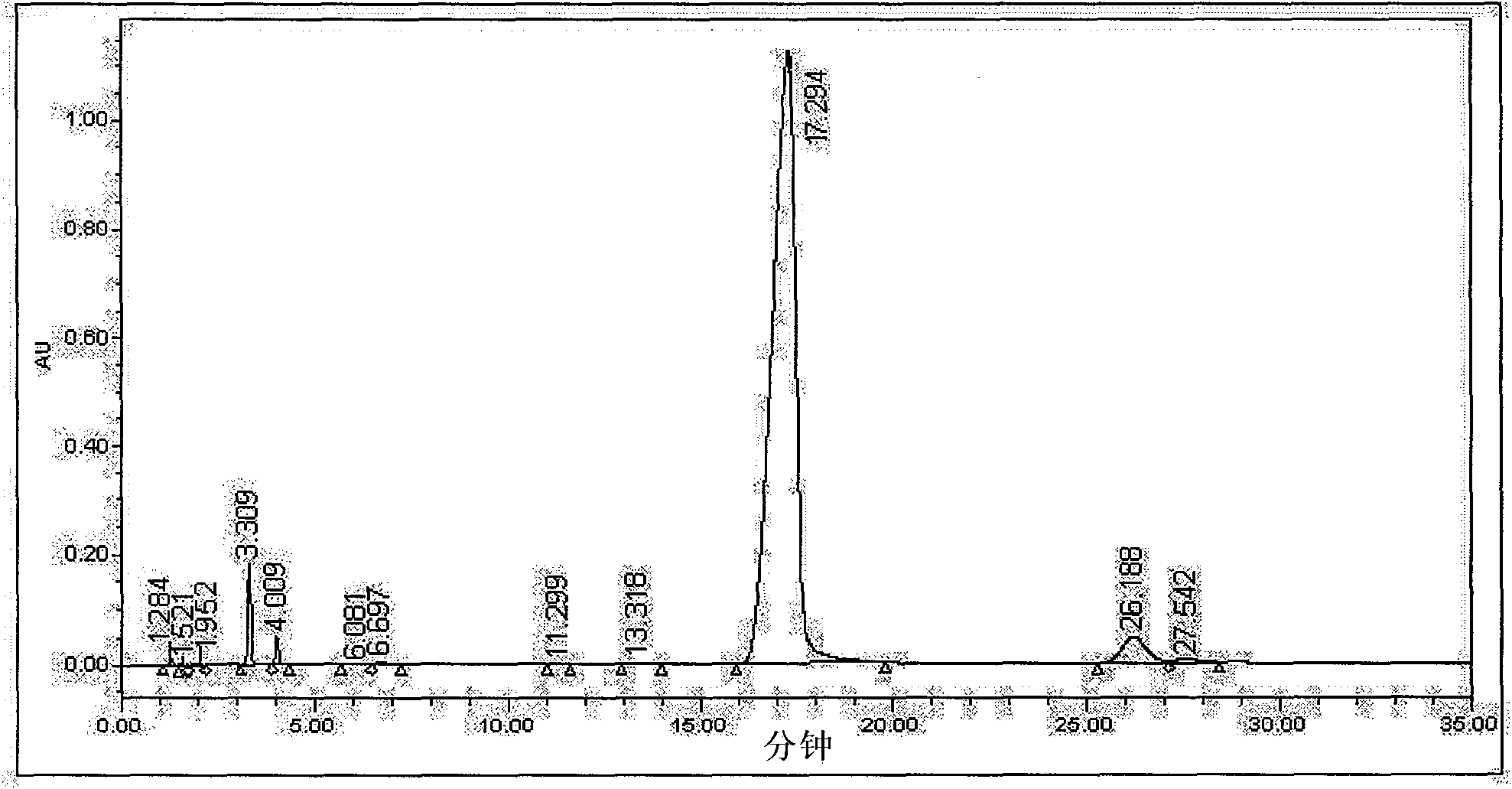
[0024] Get the cefotetan acid crude product 20g (the content of HPLC detection isomer is 4.09% for washing purification, see image 3 ), was added to a 250ml reaction flask, and then 60ml of ethanol was added, stirred, suspended and washed for 30min at 15°C, vacuum filtered, and the filter cake was detected by HPLC, and the isomer content was 3.61%. Add 60 ml of cyclohexane to the filter cake in the first step, stir and wash at the same temperature for 30 min, vacuum filter, and detect by HPLC of the filter cake, the isomer content is 2.66%. Then add 80ml methanol to the filter cake of the second step, stir and wash at the same temperature for 30min, vacuum filtration, filter cake HPLC detection, isomer content is 0.95%, see Figure 4 . The yield is about 55%.
Embodiment 3
[0026] Get the cefotetan acid crude product 20g (the content of HPLC detection isomer is 2.98% for washing purification, see Figure 5 ), was added to a 250ml reaction flask, and then 40ml of ethanol was added, stirred, suspended and washed for 30min at 15°C, vacuum filtered, and the filter cake was detected by HPLC, and the isomer content was 2.62%. Add 40ml of cyclohexane to the filter cake in the first step, stir and wash at the same temperature for 30min, vacuum filter, filter cake HPLC detection, the isomer content is 1.5%. Then add 40ml methanol to the filter cake of the second step, stir and wash at the same temperature for 30min, vacuum filtration, filter cake HPLC detection, isomer content is 0.36%, see Figure 6 . The yield is about 57%.
[0027] From the results shown in the above examples and accompanying drawings, it can be seen that the present invention can wash and purify cefotetan acid crude products with different isomer contents to meet the required purity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com