Assay kit for detecting human papillomavirus and preparation and use thereof
A human papillomavirus, detection kit technology, applied in biochemical equipment and methods, microorganism-based methods, and microbial determination/examination, etc. expensive, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Preparation method of human papillomavirus detection kit
[0058] 1.1 Design of primer pairs
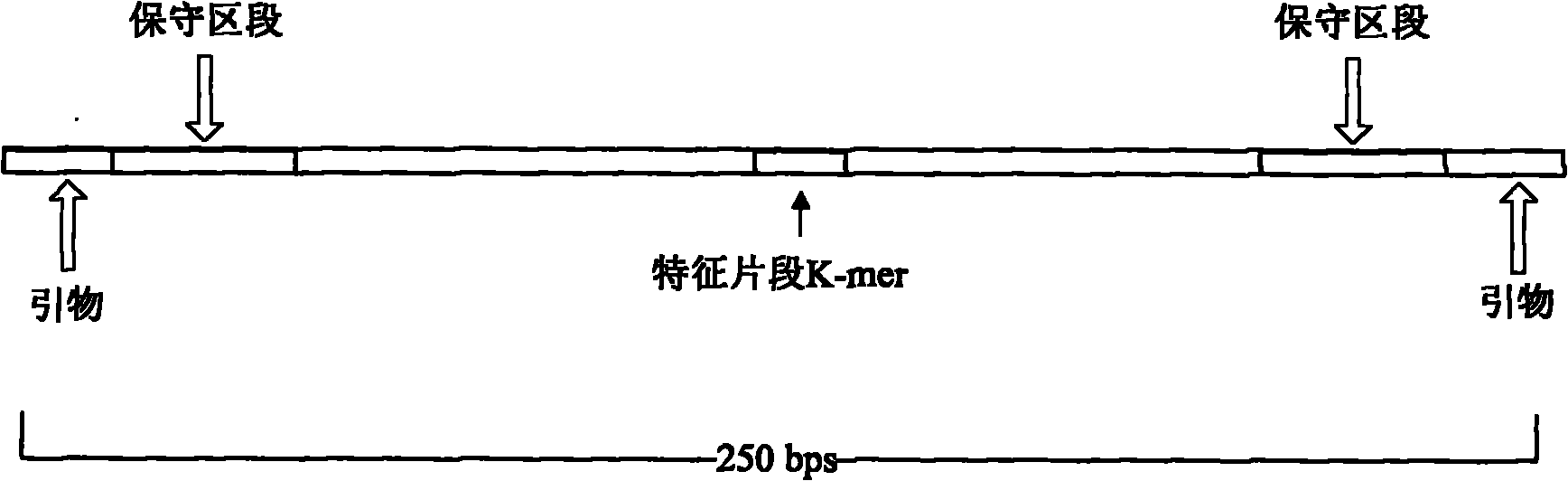
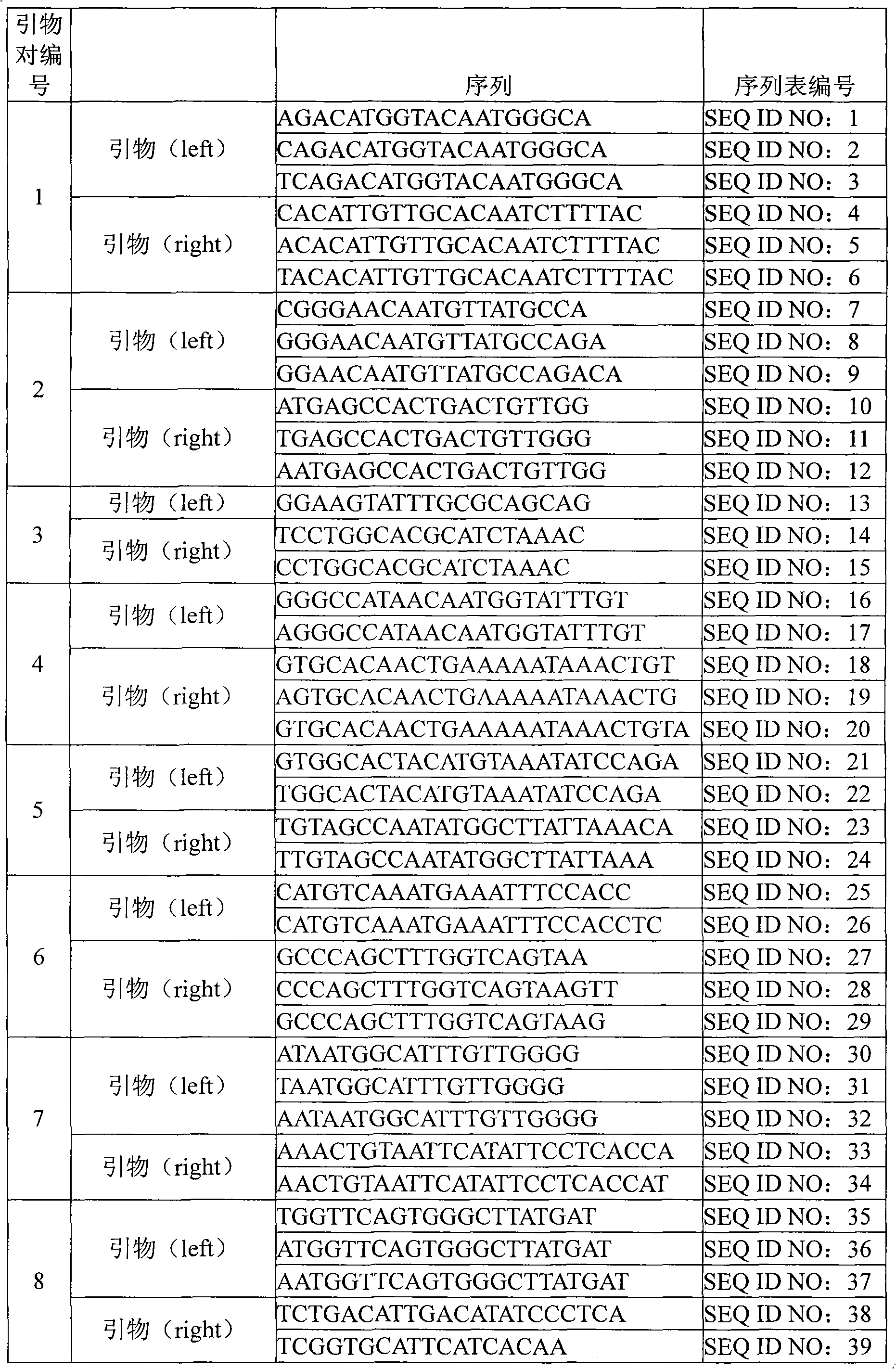
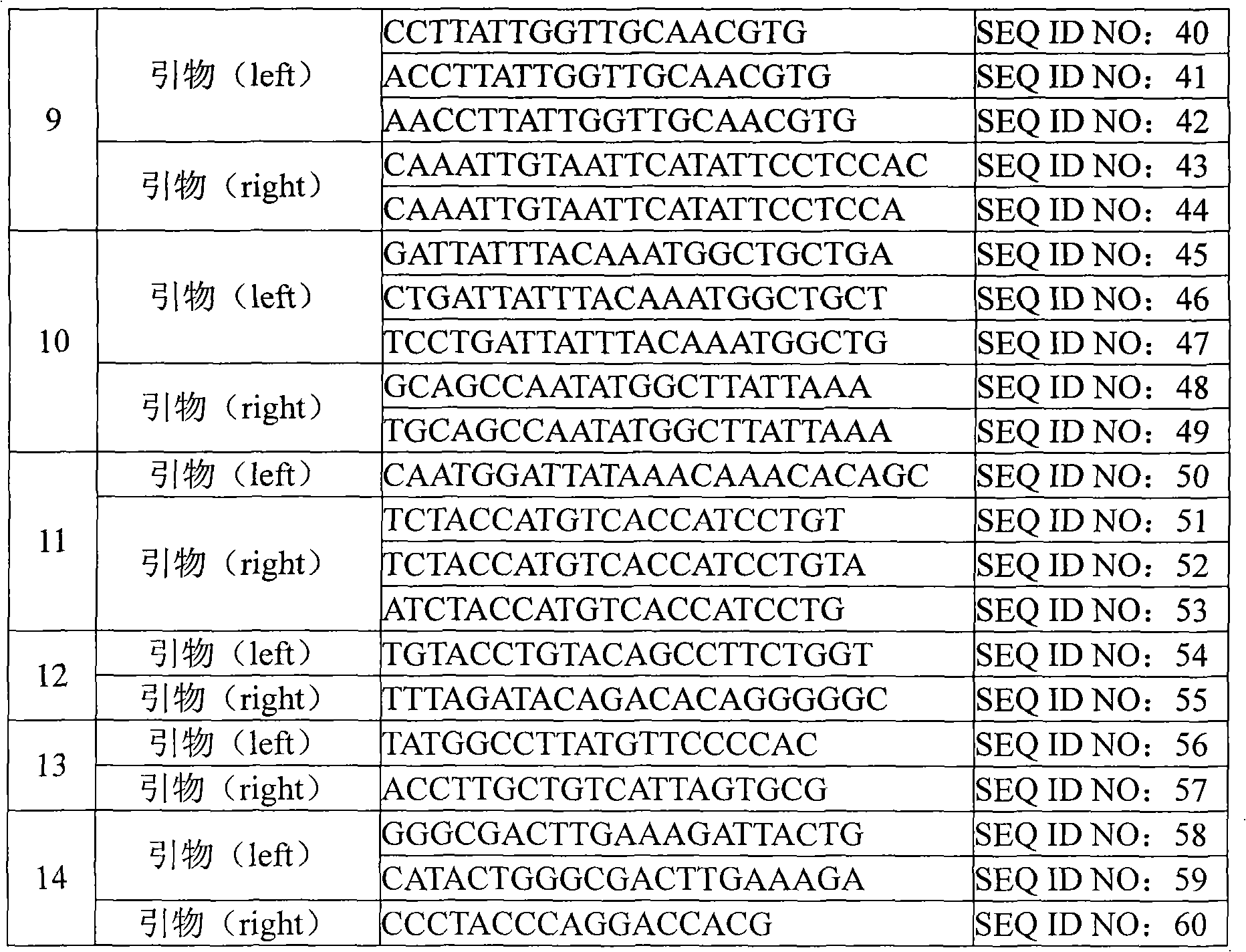
[0059] Such as figure 1 As shown in, by detecting the DNA sequence of various subtypes of human papillomavirus, the conserved and variable regions shared by different subtypes of viruses can be found through sequence comparison. Based on these data, the following primer pairs can be designed, and then synthesized Primer sequence (The primer synthesis company is Shanghai Biological Engineering Technology Service Co., Ltd.). The HPV subtype corresponding to each primer pair and the k-mer sequence corresponding to each subtype are shown in the following table:
[0060] 1. Primer pair 1
[0061]
[0062]
[0063] 2. Primer pair 2
[0064]
[0065] 3. Primer pair 3
[0066]
[0067] 4. Primer pair 4
[0068]
[0069] 5. Primer pair 5
[0070]
[0071]
[0072] 6, primer pair 6
[0073]
[0074] 7. Primer pair 7
[0075]
[0076] 8. Primer pair 8
[0077]
[0078] 9. Primer pair 9
[00...
Embodiment 2
[0098] Example 2 High-throughput use of human papillomavirus detection kit:
[0099] Specimen sources: 7 tissue samples from clinical medical units.
[0100] The kit was prepared using the method of Example 1. The difference is that when synthesizing the primers, different DNA tags are added to the 5'ends of the sequencing primers used for detecting different samples. The relationship between the DNA tags and the samples is listed in the following table:
[0101] Sample number
DNA tag sequence
1
CCAATGCA
2
CAATGCAT
3
AATGCATT
4
ATGCATTG
5
TGCATTGG
6
GCATTGGA
7
CATTGGAA
[0102] 2.1 Processing of test specimens:
[0103] 1. Add PBS or saline to the tissue sample and centrifuge to wash;
[0104] 2. Add 20-50uL of digestion lysis solution (mixed solution of 0.5% NP-40 and 0.5% tween-20), and lyse at 95-98 degrees Celsius for 15-30 minutes;
[0105] 3. After lysis, centrifuge at 12000r / min for 5-10min, and take the supernatant for use.
[0106] 2.2 PCR amplification...
Embodiment 3
[0117] Example 3 Clinical sensitivity and specificity test of human papillomavirus detection kit
[0118] 1 Specimen: 50 specimens that have been clinically classified for HPV
[0119] 2 Kit: prepared by the method of Example 1
[0120] 3 Specimen processing, reagent configuration, sample addition and PCR amplification are the same as in Example 2
[0121] 4 Test results:
[0122] The sensitivity of the human papillomavirus detection kit is 94%; the specificity of the human papillomavirus detection kit is 100%; the accuracy of the human papillomavirus detection kit is 96%. The specific test results are shown in the table below
[0123]
[0124]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com