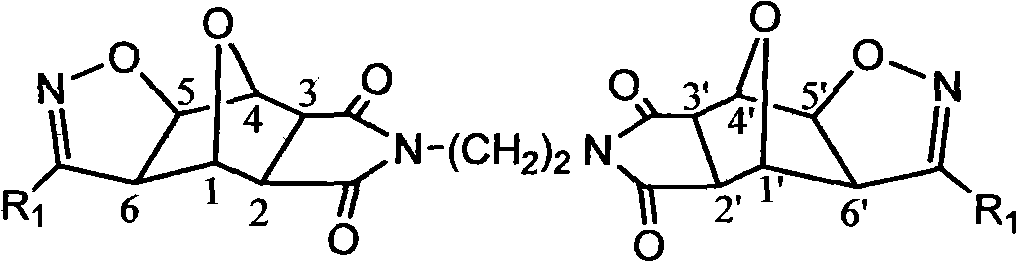
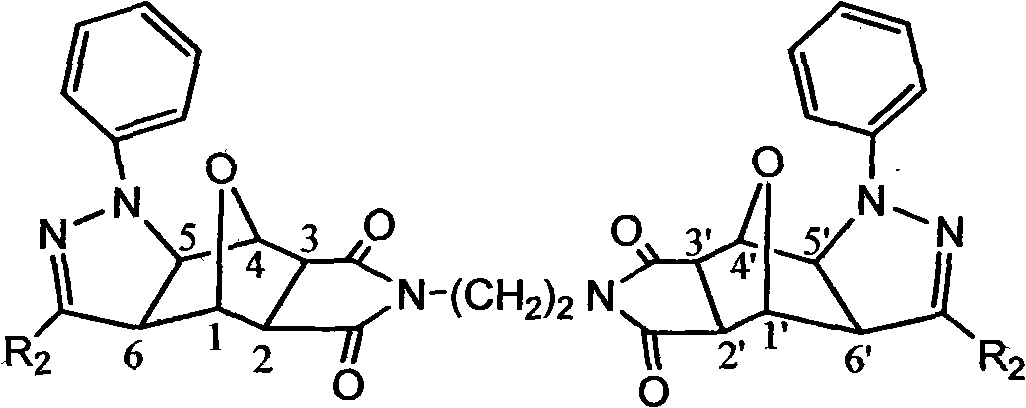
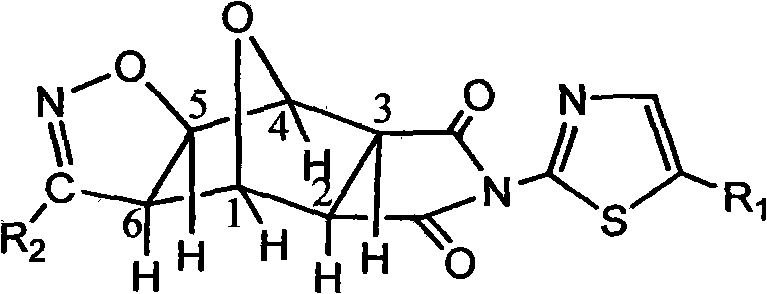
Norcantharidin derivative and activity testing method and application thereof
A technology for norcantharidin and derivatives, which is applied in biochemical equipment and methods, microbial determination/inspection, organic active ingredients, etc., can solve the problems of unsatisfactory pharmacological test results, loss of inhibitory effect, etc., and achieve good anti-tumor effects. active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: The effect of the 32 compounds on the activity of human lung adenocarcinoma cell A549 was analyzed by cell counting kit-8.
[0048] (1) The cultured A549 cells were digested with trypsin and then centrifuged, resuspended with new medium, and then planted 300 A549 cells with a volume of 50ul in each well of a 384-well plate with a liquid dispenser mutidrop;
[0049] (2) At 37°C, 5% CO 2 After culturing under the condition for 24 hours, the automatic liquid handling system Bravo was used to dispense drugs for stimulation. The treatment concentrations of various compounds were: 16uM 8uM 4uM 2uM 1uM 0.5uM 0.25uM 0.125uM, and each treatment had four repetitions;
[0050] (3) After 48 hours, use the CCK-8 kit to detect the activity of the cells: add 5ul of CCK-8 solution to each well, and use a microplate reader to read the absorption value of each well at a wavelength of 450nm; continue in the cell culture incubator Incubate for 100 min, and read the absorbance a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com