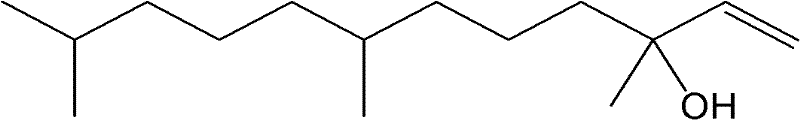
Catalyst for selective hydrogenation of 3,7,11-trimethyl-1-dodecyne-3-alcohol
An alcohol selectivity and catalyst technology, applied in physical/chemical process catalysts, hydrogenation preparation, organic chemistry, etc., can solve problems such as low selectivity, product discoloration, and odor, and reduce the content of precious metal Pd and reaction conditions. The effect of mild, excellent catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
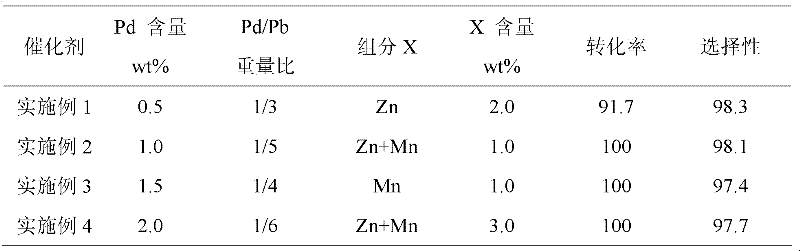
Embodiment 1-4
[0022] Catalyst preparation: take the dry calcium carbonate carrier, put it into a high-temperature furnace for roasting at 300-500°C, the heating rate is 10°C / min, and keep it for 3-4 hours after reaching the preset temperature. After natural cooling, press 1: 4-6 ratio Mix well with deionized water, slowly add PdCl 2 Hydrochloric acid solution (or PdNO 3 Solution), continue stirring the mixture at 25°C for 30min, then heat up to 85°C and continue stirring for 30min, during the stirring process, use NaOH solution to control the pH value of the solution between 5-6, during this process, the liquid phase The palladium ions are continuously adsorbed on the surface of the carrier. Centrifuge the above mixed solution to obtain a solid precipitate, mix the precipitate with deionized water at a ratio of 1:4-6, and carry out reduction treatment on the solid precipitate with sodium formate solution at 60-80°C. Then add the prepared compound solution of Pb and X source into the above...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com