Activator protein for fibrinolytic system
A technology of fibrinolysis and protein activation, applied in the fields of peptide/protein components, cardiovascular system diseases, anti-animal/human immunoglobulin, etc., can solve the problem of thrombolytic active components without further progress, earthworm medicinal function Restrictions and other issues, to achieve the improvement of hemorheology indicators, good economic value and social benefits, and promote the effect of thrombolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Separation and purification of FAP in earthworms
[0054] Materials and Methods
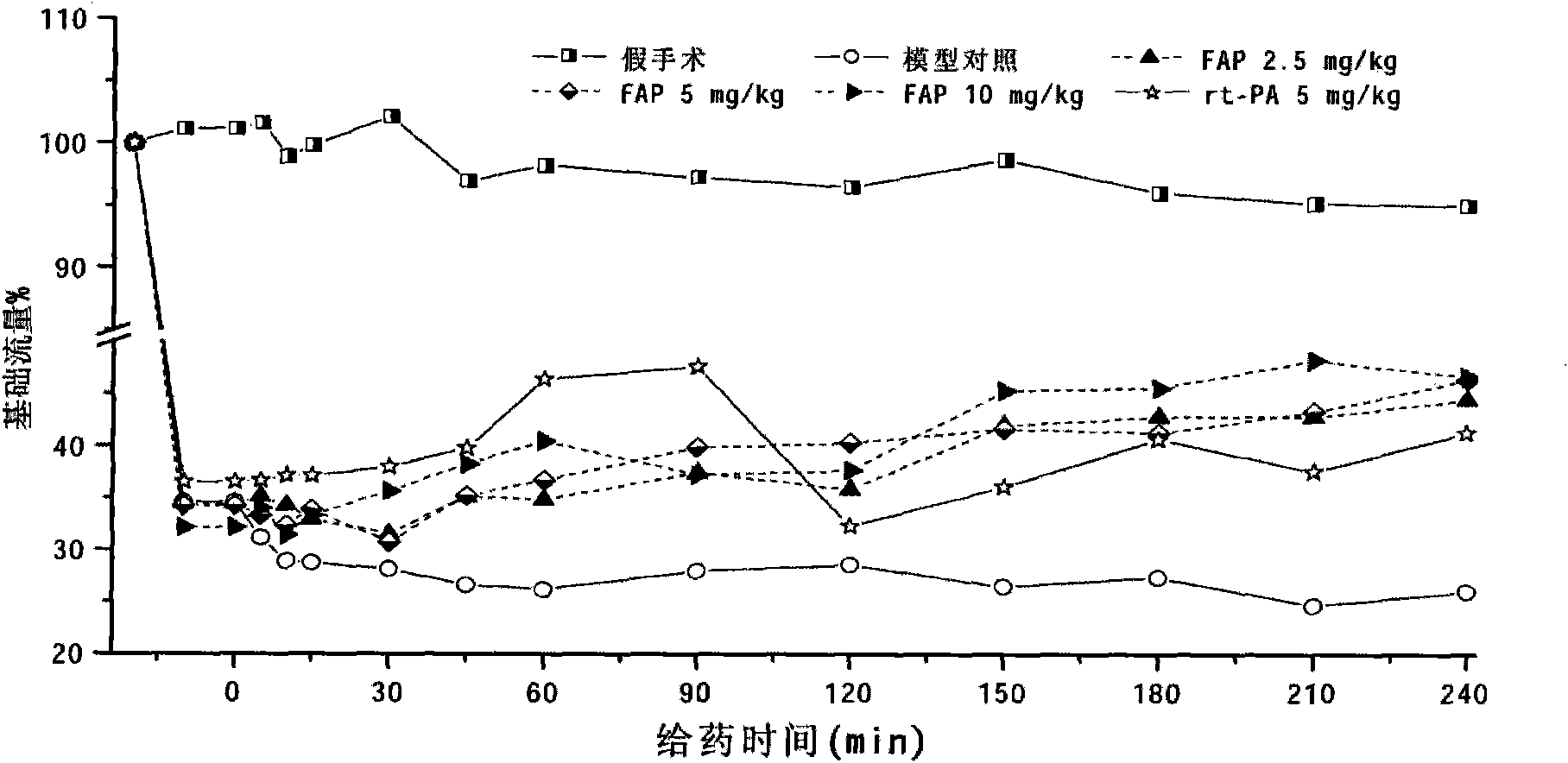
[0055] Earthworms: Assay of FAP activity of earthworms (Eisenia fortida) cultured in the suburbs of Wuxi: using Example 3 (FAP in vitro plasminase activity and plasminogen kinase activity), Example 4 (FAP on photochemically induced rats) The dissolution of focal cerebral thrombus), the method described in Example 5 (preparation and application of FAP antibody) is a detection means to measure the biological activity and immune activity of FAP.
[0056] FAP extraction: wash the fresh earthworm tissue, after homogenization, add the same amount of water, extract at 25°C for 12-14h, centrifuge at 8000r / min, supernatant is salted out with 20% and 50% ammonium sulfate, and take 50% The salting-out precipitate was dialyzed against water overnight, the dialysate was centrifuged at 17000r / min, and the supernatant was freeze-dried. Extract A is obtained.
[0057] Gel filtration: Sephadex...
Embodiment 2
[0062] In vitro recombinant expression of embodiment 2 FAP
[0063] 1. FAP gene acquisition
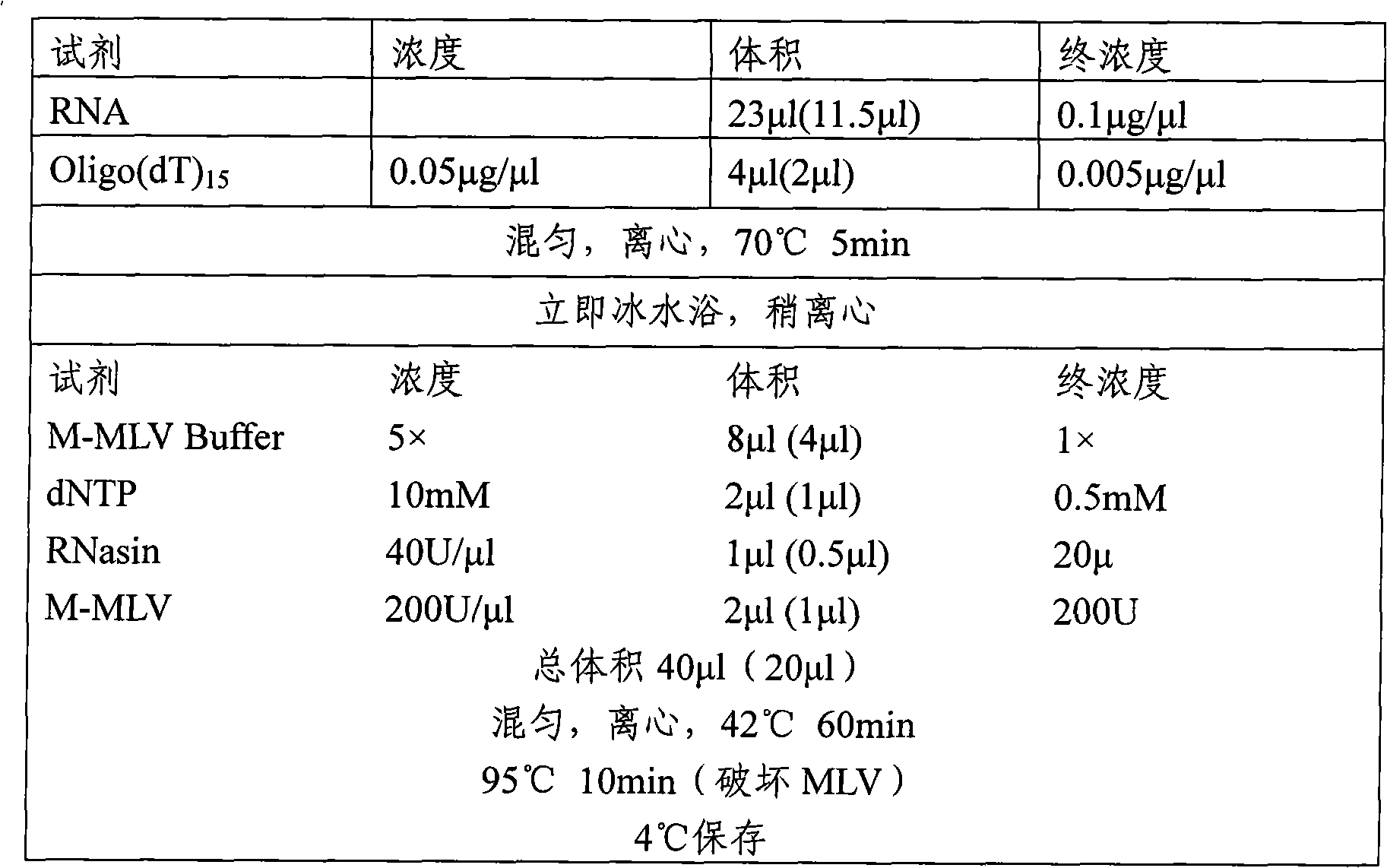
[0064] A. Earthworm mRNA Extraction
[0065] (1) Weigh 0.2g fresh earthworm (Eisenia fortida), add 1ml TRIzol reagent to prepare earthworm homogenate, and incubate at 4°C for 5min.
[0066] (2) Add 0.2ml of chloroform, close the cap tightly and shake vigorously for 15Sec, then place it on ice for 5min.
[0067] (3) 4°C, 12000×g, centrifuge for 15 minutes.
[0068] (4) Transfer the upper aqueous phase to another tube, add 0.5ml of isopropanol, and incubate on ice for 10min.
[0069] (5) Centrifuge at 12000×g for 10 minutes at 4°C.
[0070] (6) Discard the supernatant, add 1 ml of 75% ethanol to the precipitate (containing RNA) to wash, and vortex to mix.
[0071] (7) Centrifuge at 10,000×g for 5 minutes at 4°C to obtain RNA precipitates.
[0072] (8) After air drying, dissolve with appropriate amount of TE or RNase-free water for later use.
[0073] B. Primer Design
[0074] Pr...
Embodiment 3
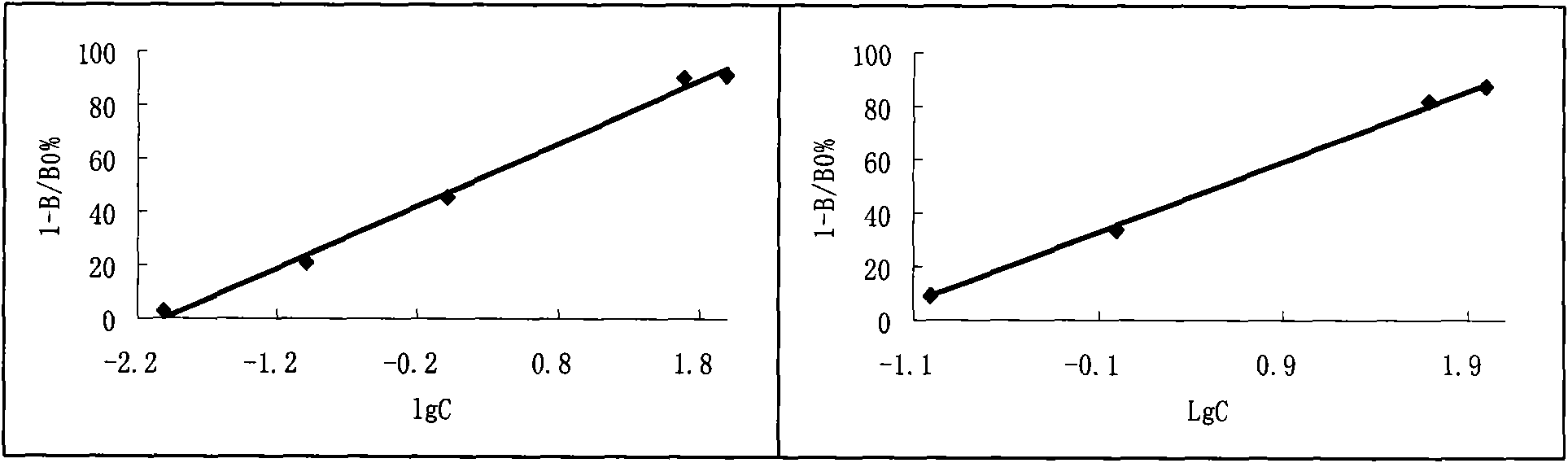
[0129] In vitro plasmin activity and plasminogen kinase activity assay of embodiment 3 FAP
[0130] Materials and Methods
[0131] Materials: FAP (purified from earthworms); plasminogen (bovine blood, each tube is equivalent to 12 casein units); thrombin (bovine blood, each tube is equivalent to 130 BP units), urokinase (each tube is equivalent to 730 units) and Fibrinogen (coagulable protein 81mg per vial) (China, National Institute for the Control of Pharmaceutical and Biological Products)
[0132] The fibrin plate method is used to measure the kinase activity and plasmin activity of FAP. According to the fibrin plate method of Doegny et al., slightly improved:
[0133] 1) Determination of plasminogen kinase activity: 200 μl plasmin stock solution + 100 μl thrombin (bovine blood). After mixing, add it to the fibrin stock solution preheated at 37°C, pour the mixed solution into the agar solution at 56°C, mix quickly, spread two flat plates with a diameter of 9 cm, place the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com