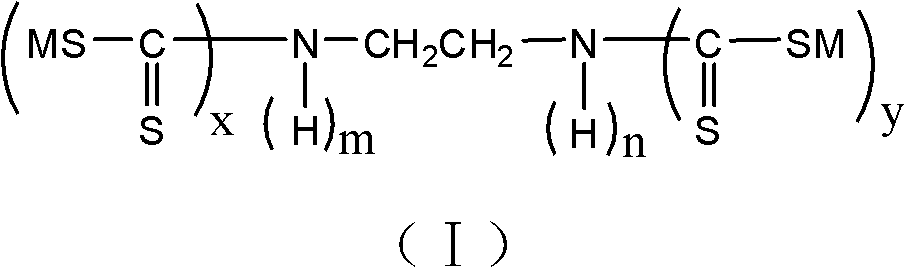Ethylenediamine-based heavy metal chelating agent and preparation method thereof
A heavy metal chelating agent, ethylenediamine-based technology, applied in chemical instruments and methods, water/sewage treatment, water/sludge/sewage treatment, etc., can solve the problem that the concentration of heavy metal ions is difficult to meet the standard discharge, and it is not suitable for large-flow heavy metal wastewater It can solve the problems of unfavorable floc growth and other problems, and achieve the effect of easy promotion, convenient storage and transportation, and good flocculation and sedimentation performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Add 0.1L ethylenediamine and 2L absolute ethanol to a 10L reactor with mechanical stirring and reflux condenser, then add 244g of granular or flaky solid NaOH with a mass percentage of 96%, and stir Add 362mL of carbon disulfide dropwise, and control the addition rate to raise the temperature of the system to 40-45°C naturally;
[0033] (2) After adding carbon disulfide, add 0.88g of hydroquinone and continue the reaction for 1h;
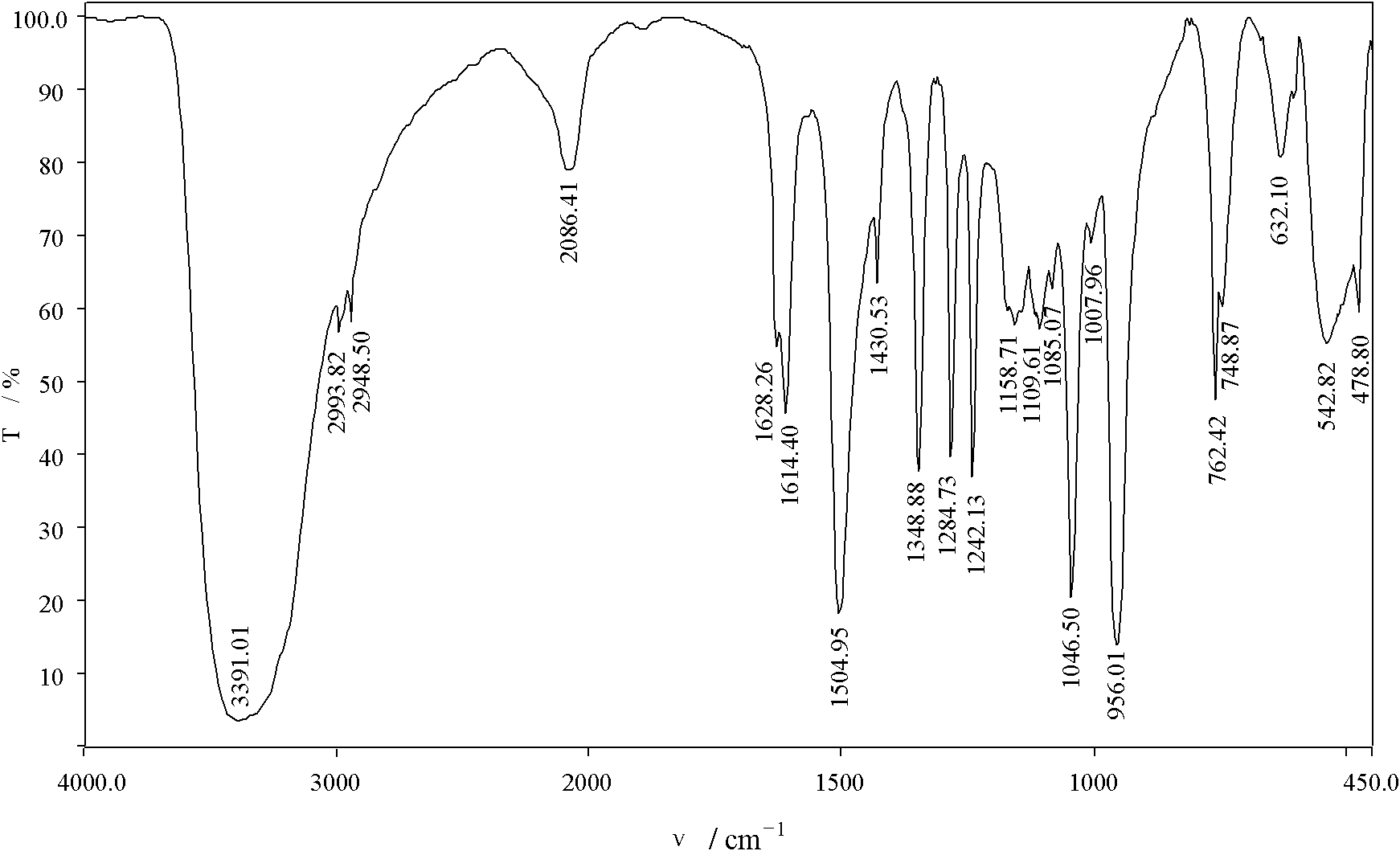
[0034] (3) Filtration, the filter cake was washed with absolute ethanol, and vacuum-dried to constant weight at 40°C to obtain a light yellow powder, i.e. the product, with a yield of 87.19%, and its sulfur content was determined to be 52.35%;
[0035] After determination, the mass percentage of each component in the product is: the compound content of structural formula (I) is 99.31%; the antioxidant content is 0.13%; the sulfur-containing compound content is 0.51%; the alkali content is 0.05%.
[0036] (4) reclaim step (3) gained filtr...
Embodiment 2
[0040] (1) Add 0.1L of ethylenediamine and the filtrate collected in step (4) of Example 1 to a 10L reactor with mechanical stirring and a reflux condenser, supplement absolute ethanol to 2.5L, and then add 244g mass percent Granular or flaky solid NaOH with a content of 96%, add 345.6mL of carbon disulfide dropwise under stirring, and control the adding speed so that the system naturally heats up to 40-45°C;
[0041] (2) After adding carbon disulfide, add 1.32g of hydroquinone and continue the reaction for 1.5h;
[0042] (3) Filtration, the filter cake was washed with absolute ethanol, and vacuum-dried to constant weight at 40°C to obtain a light yellow powder, i.e. the product, with a yield of 98.54%, and its sulfur content was determined to be 53.11%;
[0043] After determination, the mass percentage of each component in the product is: the compound content of structural formula (I) is 99.05%; the antioxidant content is 0.19%; the sulfur-containing compound content is 0.69%...
Embodiment 3
[0046] (1) Add 0.1L of ethylenediamine and the filtrate collected in step (4) of Example 2 to a 10L reactor with mechanical stirring and a reflux condenser, supplement absolute ethanol to 3.0L, and then add 244g mass percent Granular or flaky solid NaOH with a content of 96%, add 362mL of carbon disulfide dropwise under stirring, control the adding speed to make the system naturally warm up to 40-45°C;
[0047] (2) After adding carbon disulfide, add 1.76g of hydroquinone and continue the reaction for 2h;
[0048] (3) Filtration, the filter cake was washed with absolute ethanol, and vacuum-dried to constant weight at 40°C to obtain a light yellow powder, i.e. the product, with a yield of 99.07%, and its sulfur content was determined to be 53.31%;
[0049] After determination, the mass percentage of each component in the product is: the compound content of structural formula (I) is 98.53%; the antioxidant content is 0.25%; the sulfur-containing compound content is 1.15%; the alk...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com