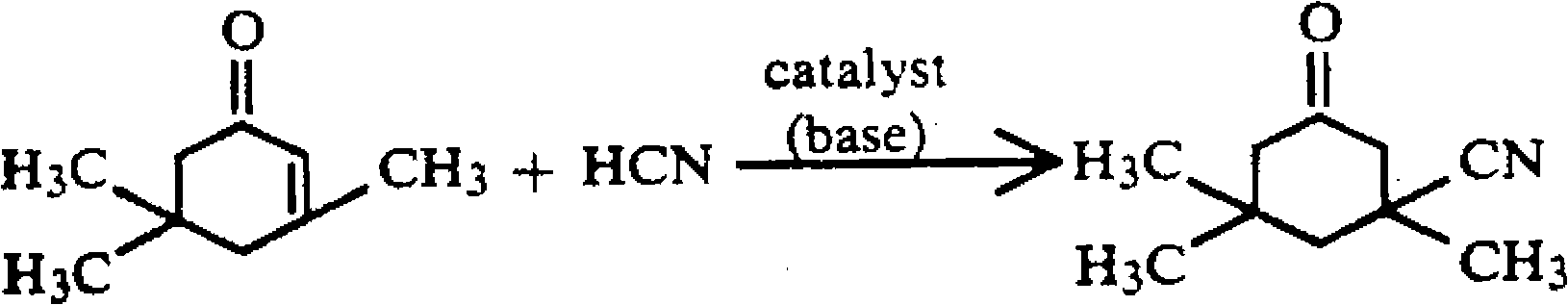Method for preparing 3-cyan-3,5,5-trimethyl cyclohexanone
A technology of isophorone nitrile and isophorone, applied in hydrogen cyanide addition preparation, organic chemistry and other directions, can solve the problems of reduced rectification yield, impact on economic benefits, and high requirements for production equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 276.4g (2.0mol) of isophorone to a four-neck flask equipped with a stirring, thermometer, and reflux condenser, raise the temperature to 100°C, add 2.0g of lithium hydroxide, and dropwise add 27.0g (1.0mol) of hydrocyanic acid , the dropping time is about 50 minutes, keep the temperature at 170° C., keep the temperature for 30 minutes after dropping, cool down to 100° C., add 10 g of p-toluenesulfonic acid, and sample and analyze the yield of isophorone nitrile is 99% (calculated as HCN ), after recovering 137.1 g of isophorone by distillation, 150 g of methanol and 3.5 g of activated carbon were added, refluxed for decolorization, filtered, cooled to -5°C for crystallization. Filter and dry to obtain 150.2 g of light yellow crystalline isophorone nitrile product with a content of 99.3% and a yield of 90.3% (calculated as HCN).
Embodiment 2
[0023] The operation was the same as in Example 1. After recovering 137.4 g of isophorone by distillation, 150 g of industrial ethanol and 3.5 g of activated carbon were added, refluxed for decolorization, filtered, cooled to -5°C for crystallization. filtered, dried to obtain light yellow).
Embodiment 3
[0025] The operation was the same as in Example 1. After recovering 137.4 g of isophorone by distillation, 150 g of dichloroethane and 3.5 g of activated carbon were added, refluxed for decolorization, filtered, and cooled to -5°C for crystallization. After filtering and drying, 150.0 g of a yellow crystal isophorone nitrile product was obtained, with a content of 99.0% and a yield of 89.9% (calculated as HCN).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com