Novel nitrone ligand, organic metal iridium catalyst and preparation method and application thereof
An organometallic and organic compound technology, applied in organic chemistry methods, preparation of organic compounds, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of low synthesis yield, etc. The effect of high catalytic activity and high reaction yield
Active Publication Date: 2010-10-06
JIANGSU SINOCOMPOUND CATALYST
View PDF2 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
At present, in many chemical synthesis reactions, it is necessary to extend or branch the carbon chain, and the synthesis yield is not high by conventional methods, and the requirements for the selection of reactants are relatively high, and the present invention comes from this
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
experiment example 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
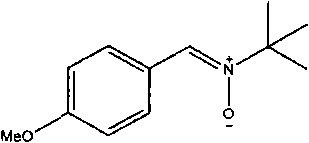
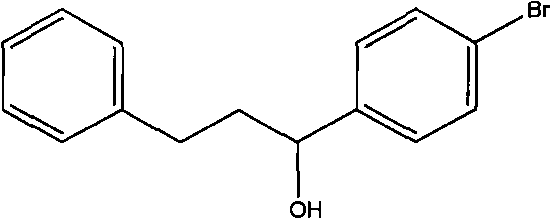
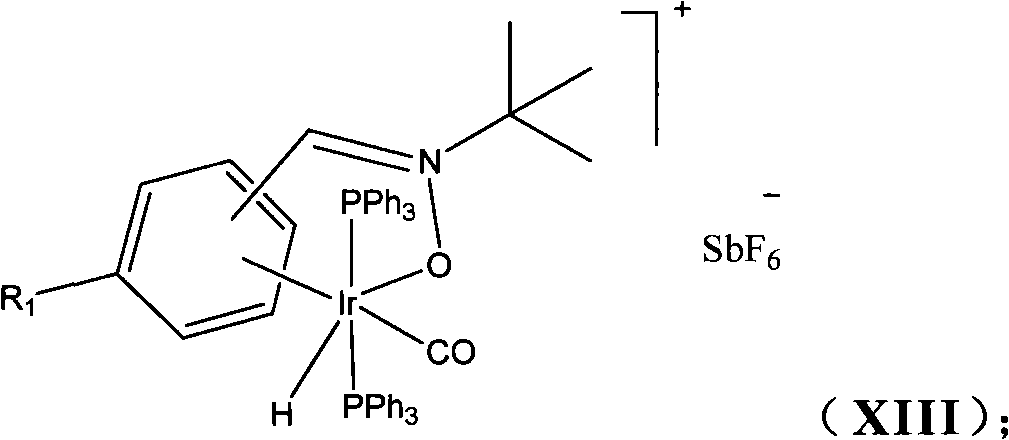
The invention discloses an organic compound, which is characterized in that: the compound has the structure of a formula (I): RAn (I); A has a nitrone ligand with the structure of a formula (II); n is 1 or 2; R is selected from one of the following aryl groups: phenyl group or naphthyl group, and R is substituted by one or more of the following groups: hydrogen, nitro group, trifluoromethyl group, halogen, methyl group, methoxy group, hydroxy group, phenyl group, phenolic group and isopropyl group. The compound can be synthesized into a series of catalysts with high catalytic activity and a high turnover rate, various easy-obtained, economic substituted Alpha-phenethyl alcohol compound and phenethyl alcohol compound can be catalyzed under mild conditions to react to prepare various alcohol compounds, the reaction can obtain a high reaction yield in a short reaction time, meanwhile, almost no byproducts are produced, and therefore the reaction is environment-friendly green catalytic reaction and atomic economic catalytic reaction.
Description
technical field The invention belongs to the technical field of organic chemistry synthesis, and specifically relates to a novel nitrone ligand, an organometallic iridium catalyst and a preparation method thereof, and the application of the organometallic iridium catalyst in the synthesis of organic compounds to form carbon-carbon bonds Background technique Carbon-hydrogen bond activation is not only a challenge at the frontiers of organic chemistry science, but will also be at the heart of a new generation of material transformations. However, due to the similar electronegativity of carbon atoms and hydrogen atoms, the carbon-hydrogen bond presents the basic structural characteristics of relatively stable and low polarity. A chemical transformation takes place, so the first problem encountered in this regard is its low reactivity. For relatively complex organic compounds, there are many different carbon-hydrogen bonds in the same molecule, so how can we realize the transfo...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07C251/48C07F19/00C07C249/08B01J31/24C07B37/00C07C43/23C07C41/30C07C33/18C07C29/34C07C33/46C07C33/20
Inventor 赵劲钱勇李娟
Owner JIANGSU SINOCOMPOUND CATALYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com