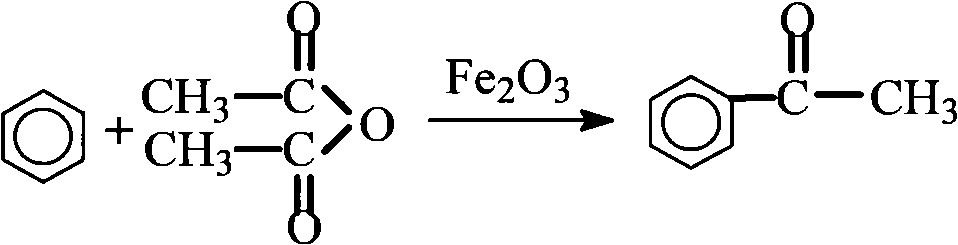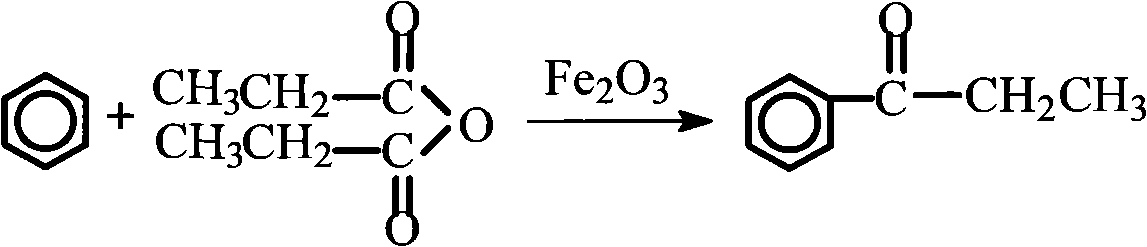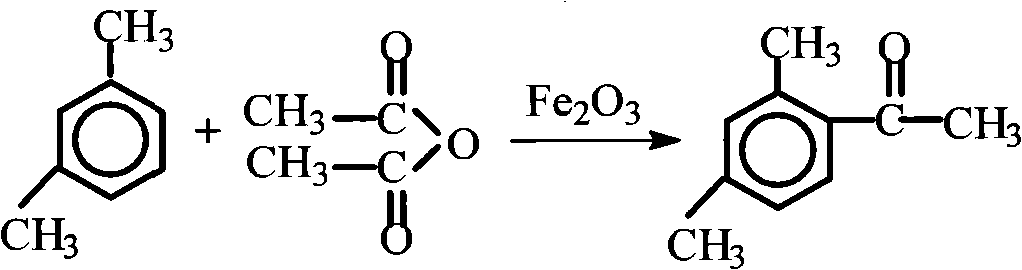Improved method for preparing aromatic ketone
A technology of aromatic ketones and acid anhydrides, which is applied in the direction of condensation preparation of carbonyl compounds and organic chemistry, etc., can solve the problems of affecting the purity of aromatic ketone products, affecting the aromatic ketone intermediates, and complex post-treatment processes, achieving good texture, little environmental pollution, Post-processing simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 synthetic aromatic ketone-acetophenone
[0031] (1) Raw material ratio
[0032] The mol ratio of benzene and catalyst ferric oxide is 1: 0.0007;
[0033] The molar ratio of benzene to acetic anhydride is 1: 0.67;
[0034] (2) The main reaction formula
[0035]
[0036] (3) The specific preparation steps are as follows:
[0037] ①In a 500ml dry reactor equipped with a stirring device, dropping funnel and condenser, put 160mg (0.001mol) of catalyst ferric oxide and 150ml (about 1.5mol) of benzene, heat together to 80°C while stirring, drop Add 102g of acetic anhydride (about 1mol), drop it within 1h, keep the temperature at 80°C and continue the reaction for 2h, then stop the reaction;
[0038] 2. After removing the solid catalyst ferric oxide from the reaction product obtained in step 1., wash with water and saturated soda ash solution to neutrality;
[0039] 3. the step 2. gained reaction product is dried, then atmospheric pressure distillation remo...
Embodiment 2
[0041] Embodiment 2 synthetic aromatic ketone-propiophenone
[0042] (1) Raw material ratio
[0043] The mol ratio of benzene and catalyst ferric oxide is 1: 0.0005;
[0044] The molar ratio of benzene and propionic anhydride is 1: 0.5;
[0045] (2) The main reaction formula
[0046]
[0047] (3) The specific preparation steps are as follows:
[0048] ①In a 500ml dry reactor equipped with a stirring device, dropping funnel and condenser, put 160mg (0.001mol) of catalyst ferric oxide and 200ml (2mol) of benzene, heat together to 80°C while stirring, add 130g dropwise Propionic anhydride (1mol), drop it within 1h, keep the temperature at 80°C and continue the reaction for 3h, then stop the reaction;
[0049] 2. After removing the solid catalyst ferric oxide from the reaction product obtained in step 1., wash with water and saturated soda ash solution to neutrality;
[0050] 3. step 2. the gained reaction product is dried, then atmospheric pressure distillation removes re...
Embodiment 3
[0052] Embodiment 3 synthesis aromatic ketone-2,4-dimethylacetophenone
[0053] (1) Raw material ratio
[0054] The mol ratio of m-xylene and catalyst ferric oxide is 1: 0.00033;
[0055] The molar ratio of m-xylene and acetic anhydride is 1: 0.67;
[0056] (2) The main reaction formula
[0057]
[0058] (3) The specific preparation steps are as follows:
[0059] ①In a 500ml dry reactor equipped with a stirring device, dropping funnel and condenser, put 80mg (0.0005mol) of catalyst ferric oxide and 156ml (1.5mol) of m-xylene, and heat together to 137°C while stirring, Add 102 g of acetic anhydride (1 mol) dropwise, and finish the drop within 0.5 h, keep the temperature at 137°C and continue the reaction for 0.5 h, then stop the reaction;
[0060] 2. After removing the solid catalyst ferric oxide from the reaction product obtained in step 1., wash with water and saturated soda ash solution to neutrality;
[0061] 3. step 2. the gained reaction product is dried, then atm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com