Fexofenadine hydrochloride orally disintegrating tablet and preparation method thereof
A technology of fexofenadine hydrochloride and oral disintegrating tablets, applied in the field of medicine, can solve the problems of bitter taste, reduced patient compliance, large bioavailability error, etc.
Active Publication Date: 2013-04-17
FUXIN LONGRUI CHEM CO LTD
View PDF3 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0010] Chinese patent CN10113980A discloses a pharmaceutical composition of fexofenadine hydrochloride, which uses fexofenadine hydrochloride as a raw material and uses a high-efficiency disintegrant as an auxiliary material to prepare an immediate-release preparation. This patent provides a high-efficiency The disintegrating agent is a technical inspiration for the preparation of orally disintegrating tablets through internal and external addition. Although it meets the dissolution evaluation indicators specified in the Pharmacopoeia, the best fexofenadine hydrochloride oral disintegration has not been optimized according to the characteristics of orally disintegrating tablets. tablet (such as 1 minute disintegration in the oral cavity, very bitter taste, etc.), and there is no flavoring agent in the prescription, which will reduce the patient's compliance; US Patent 995975 discloses a fexofenadine orally disintegrating tablet , the patent gives the technical inspiration of using 3%-15% tablet weight disintegrant, but its evaluation index is the dissolution index of hydrochloric acid as the medium, although it can meet the requirements, but using hydrochloric acid as the dissolution medium is not compatible with Therefore, the patent does not optimize the prescription of the best oral disintegrating agent for the properties of the raw material fexofenadine hydrochloride
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
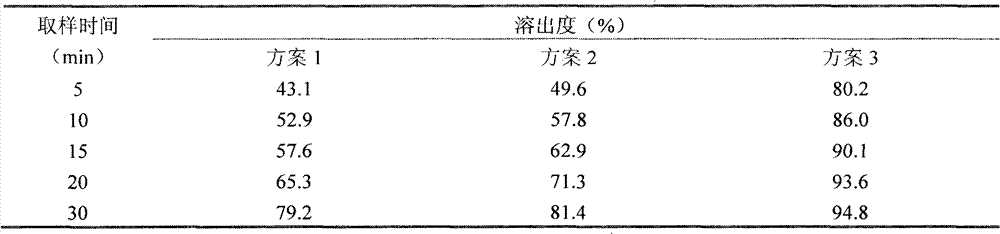
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0210]
Embodiment 2
[0212]
Embodiment 3
[0214]
[0215]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
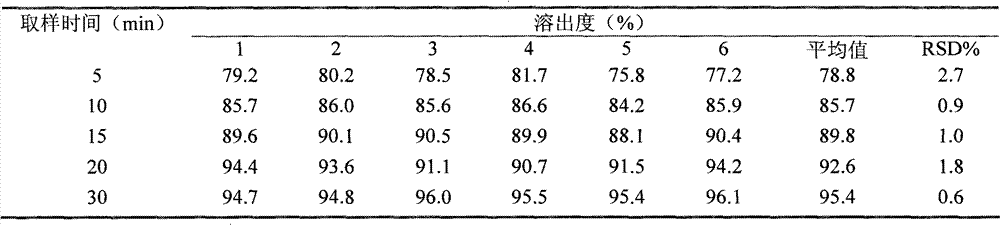
The invention discloses a fexofenadine hydrochloride orally disintegrating tablet, which consists of fexofenadine hydrochloride, a composite disintegrating agent, a filling agent, a lubricating agent and a composite flavoring agent. The fexofenadine hydrochloride orally disintegrating tablet is characterized in that the composite disintegrating agent is a highly efficient disintegrating agent, the highly efficient disintegrating agent is added internally and externally; and the composite flavoring agent is also added internally and externally. The orally disintegrating tablet of the inventionhas more excellent quality, can completely disintegrate in the oral cavity within 30 seconds, and has no gravel sense and no bitterness, and the dissolution can be more than 80 percent when determining the dissolution with water as the medium for ten minutes.
Description
technical field [0001] The invention relates to the technical field of medicine, in particular to a fexofenadine hydrochloride orally disintegrating tablet. [0002] The "high-efficiency disintegrant" in the present invention refers to an excipient added to the tablet to overcome the binder or the physical force required for tablet compression, and to promote the rapid disintegration of the tablet into small particles. [0003] The "internal addition method" in the present invention refers to the addition of pharmaceutical excipients before granulation during preparation. [0004] The "external addition method" in the present invention refers to the addition of pharmaceutical excipients to the sized dry granules during the preparation of the preparation. [0005] The "internal and external addition" in the present invention means that the pharmaceutical excipients are added according to the internal and external addition. [0006] "Composite" in the present invention refers ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): A61K9/20A61K31/445A61K47/38A61K47/34A61P37/08A61K47/32A61K47/36
Inventor 顾群金治刚孙学伟王东徐春霞
Owner FUXIN LONGRUI CHEM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com