Human N-terminal pro-B-type natriuretic peptide (NT-proBNP) immunoassay kit and preparation method thereof
An immunoassay and natriuretic peptide technology, applied in the field of immunoassay medicine, can solve the problems of expensive instruments, radioactive contamination, and need, and achieve the effects of easy operation and production, improved sensitivity, and guaranteed sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
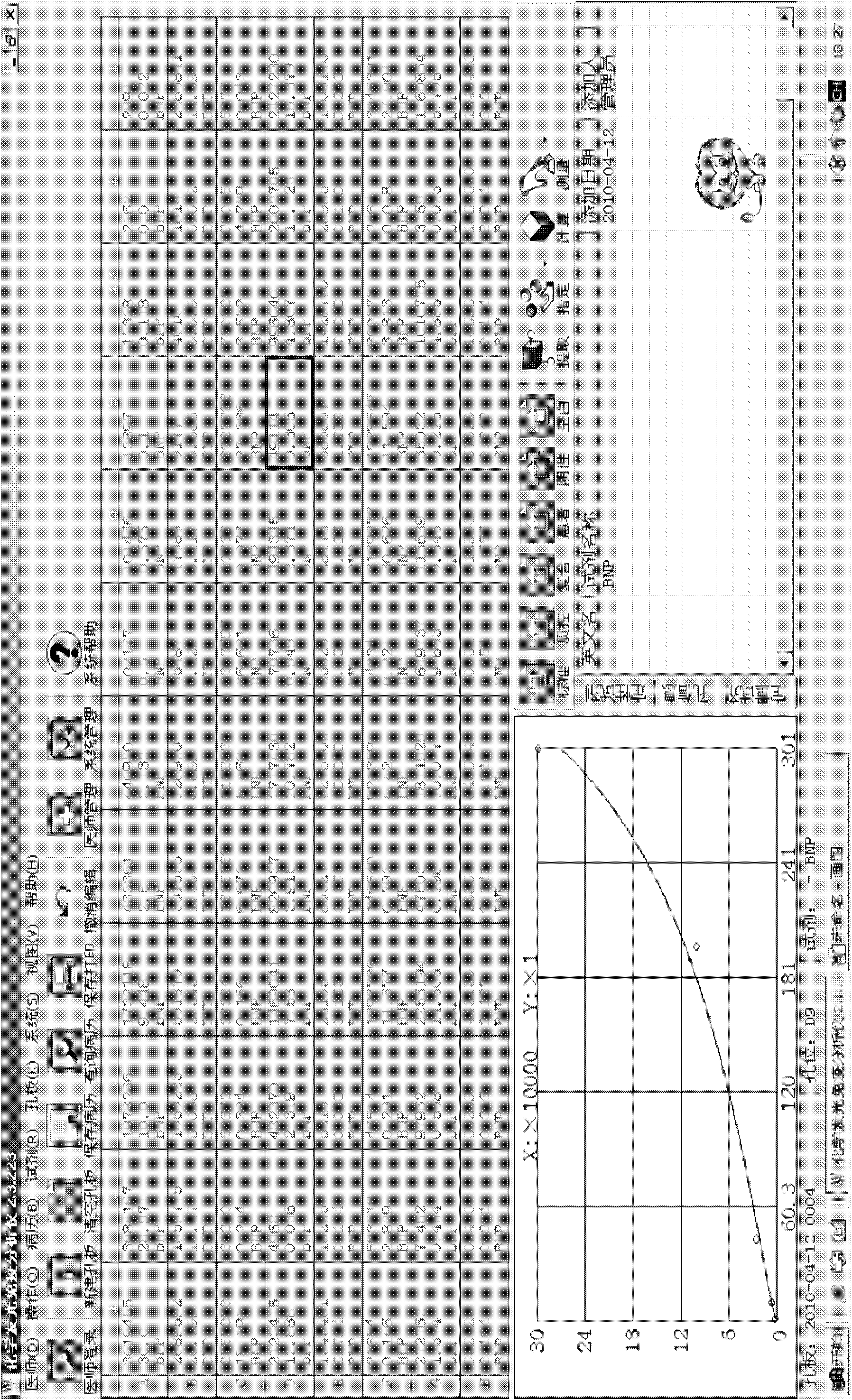
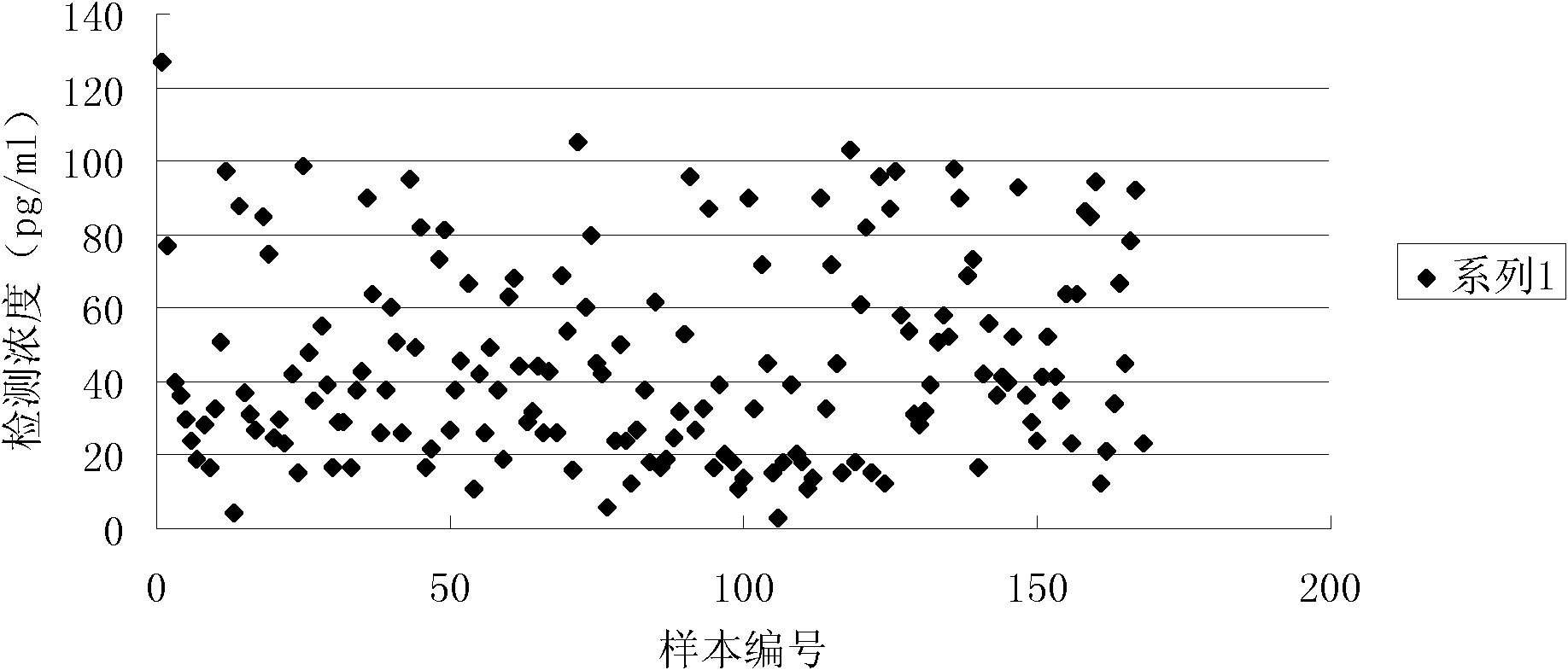
[0040] Example 1 Preparation of the inventive plate-type N-terminal pro-B-type natriuretic peptide quantitative detection kit
[0041] 1. Preparation of anti-NT-proBNP antibody
[0042] The NT-proBNP protein expressed recombinantly in our laboratory (disclosed in the patent application with publication number CN 101230101A) was taken out from the -80°C refrigerator, dissolved and filtered, and the protein content was determined by the Lowry method, using 20mmol / L PBS with pH 5.8 It was diluted to 0.5 mg / ml. Ten female Balb / c mice aged 6 weeks and weighing about 20 g were selected. Antigen emulsification adopts double syringe mutual pushing method. For the first immunization, the recombinantly expressed NT-proBNP protein was emulsified and mixed with an equal volume of Freund's complete adjuvant, and each mouse was injected intradermally and intraperitoneally at multiple points in the amount of 100 μg NT-proBNP. The second and third immunizations were carried out on the 14th...
Embodiment 2
[0091] Example 2 Preparation of the tubular human N-terminal pro-B-type natriuretic peptide quantitative detection kit of the present invention
[0092] Divide the horseradish peroxidase-labeled NT-proBNP monoclonal antibody, and prepare the chemiluminescent substrate, coating solution and blocking solution under the same conditions, except that plastic beads and plastic tubes are used as carriers, and the rest are the same as in Example 1. The same method was used to prepare the human N-terminal pro-B-type natriuretic peptide quantitative detection kit.
Embodiment 3
[0093] Example 3 Preparation of the magnetic particle-based human N-terminal pro-B-type natriuretic peptide quantitative detection kit of the present invention
[0094] The human N-terminal pro-B-type natriuretic peptide quantitative detection kit was prepared in the same manner as in Example 1 except that magnetic particles were used as the carrier.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com