Compound lidocaine emulsifiable paste and preparation method thereof
A lidocaine and cream technology, applied in ointment delivery, pharmaceutical formula, emulsion delivery, etc., can solve problems such as unstable product quality, long production cycle, and low production efficiency, so as to save equipment investment and enhance safety sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
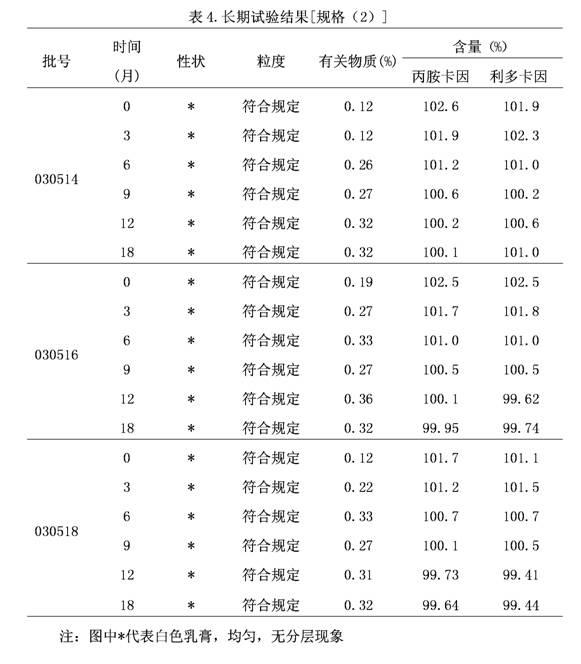
[0098] A compound lidocaine cream: every 10 kg contains the following components by weight:
[0099] Lidocaine 50g~1000g;
[0100] Prilocaine 50g~1000g;
[0101] Polyoxyethylene hydrogenated castor oil 10g~500g;
[0102] Carbomer 10g~300g;
[0103] Sodium hydroxide 40g~200g;
[0104] Purified water was added to 10kg.
[0105] The best embodiment of compound lidocaine cream: 10 kg contains the following components by weight:
[0106] Lidocaine 250g;
[0107] Prilocaine 250g;
[0108] Polyoxyethylene hydrogenated castor oil 190g;
[0109] Carbomer 934NF 100g;
[0111] Add purified water to 10kg;
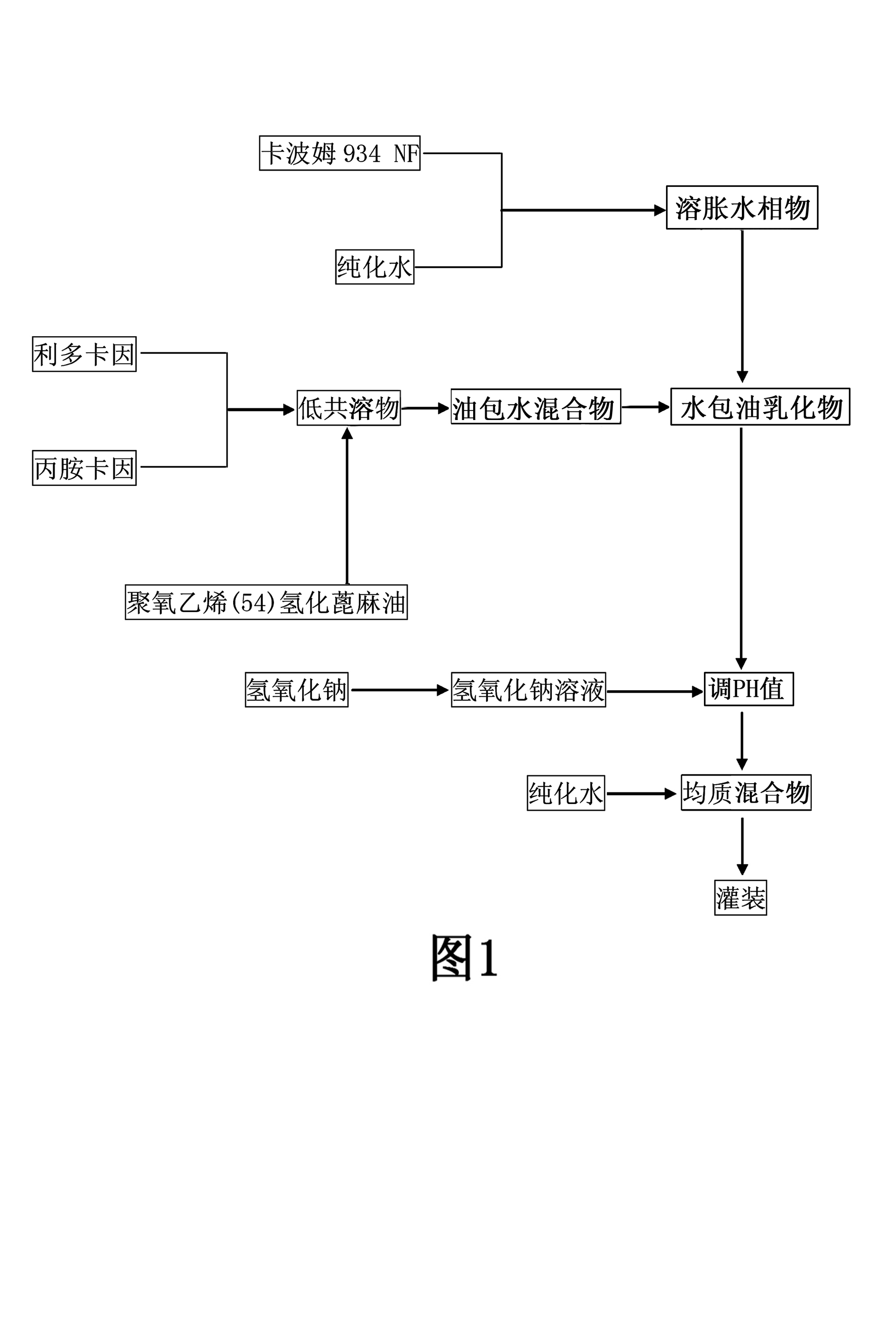
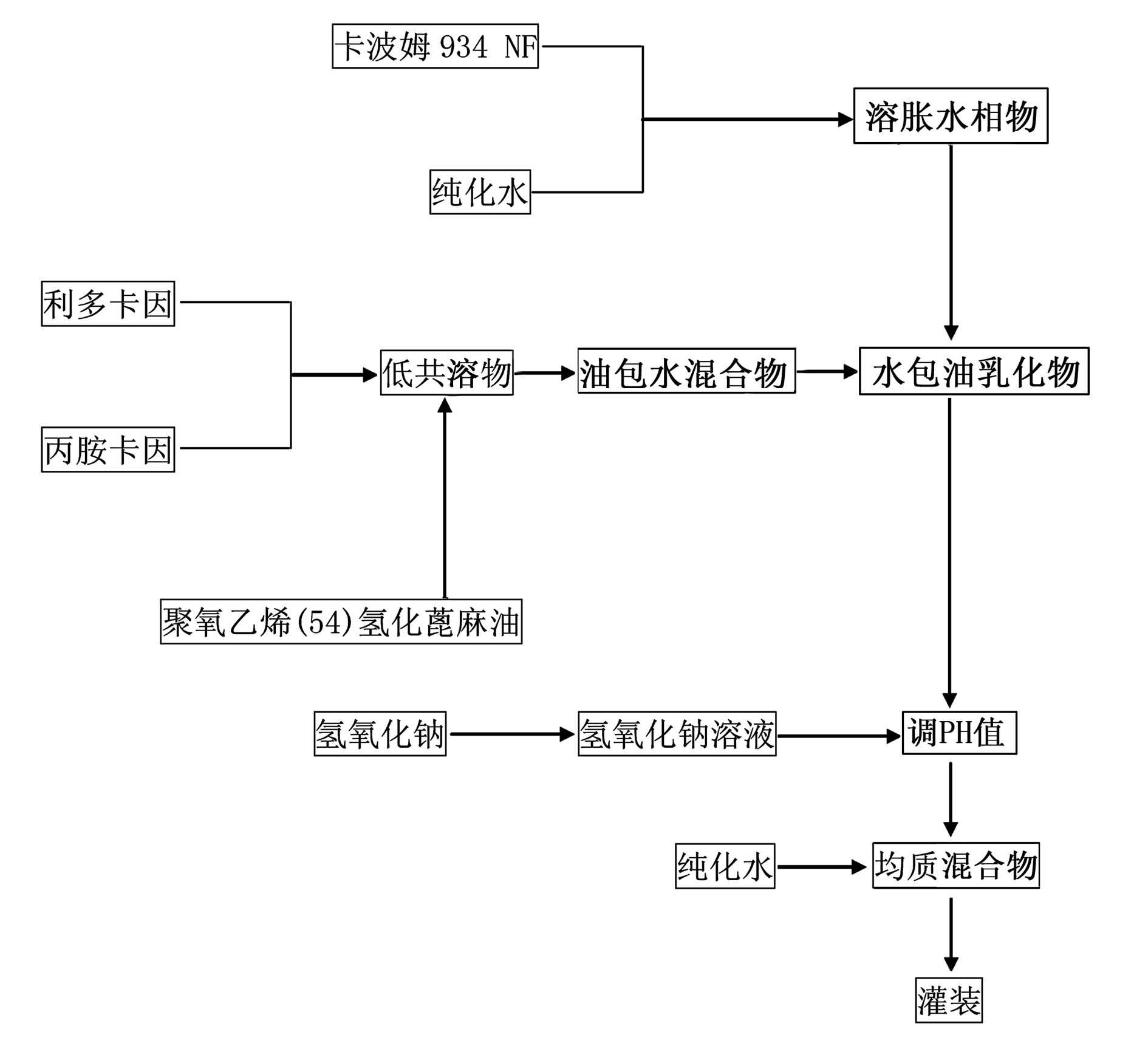
[0112] For the working process of the present invention, see figure 1 Shown: A preparation method of compound lidocaine cream, the steps are as follows:
[0113] Step 1. Pre-soak Carbomer 934 with purified water for at least 10 hours;
[0114] Step 2: Put the base lidocaine and the base prilocaine into a stirring vessel at a weight ratio of 1:1, and stir and mix at room tem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com