Preparation method of endogenous interferon inducer injection and nanometer microencapsulation solution
A technology for injections and solutions, applied in drug combinations, cardiovascular system diseases, antiviral agents, etc., can solve problems such as inconvenient administration, virus recombination, mutation, etc., to avoid strong species specificity and reduce drug residues Harm, effect of low effective dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
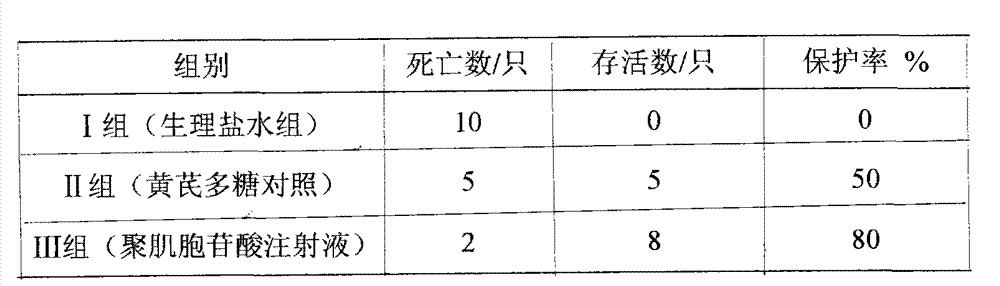
[0018] Embodiment 1: 30 weaned piglets with similar birth age and body weight were selected as experimental animals to test the prevention and treatment effect of polyinosinic acid injection on classical swine fever virus infection. The experimental animals were randomly divided into three experimental groups, 10 animals in each group. Grouping and test methods are as follows: Group I: blank control group (normal saline group); Group II: astragalus polysaccharide drug control group (astragalus polysaccharide injection, 20 mg per kg body weight); Group III: polyinosinic acid drug control group (Polyinosinic acid injection, 0.05mg per kilogram of body weight). The weaned piglets were grouped and raised in isolation according to the above experiments, and the feeding conditions were kept the same. After feeding for 1 week, each group received basic immunization with attenuated classical swine fever vaccine. After 21 days of immunization, each group was treated with drugs accord...
Embodiment 2
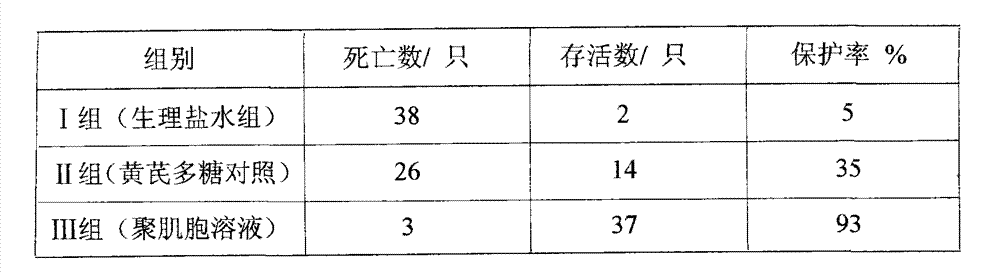
[0020]Example 2: 120 AA broilers were selected as experimental animals to test the protective effect of polyinosinic acid solution on Newcastle disease virus infection. The chicks used in the experiment were hatched and brooded until they were 7 days old, and they were given basic immunization with eye drops and nasal drops of attenuated Newcastle disease vaccine. Then the test chicks were randomly divided into 3 test groups, 40 in each group. Group I: blank control group (normal saline group); Group II: astragalus polysaccharide drug control group (astragalus polysaccharide oral liquid, 40 mg per kilogram of body weight); Group III: polyinosinic acid solution test group (polyinosinic acid Solution, 0.05mg per kilogram of body weight); the above chicks were kept in isolation cages, and the feeding conditions were kept consistent. 10d after immunization, each group will be treated with drugs according to the grouping plan, and will be challenged after 12h (challenge 10 times L...
Embodiment 3
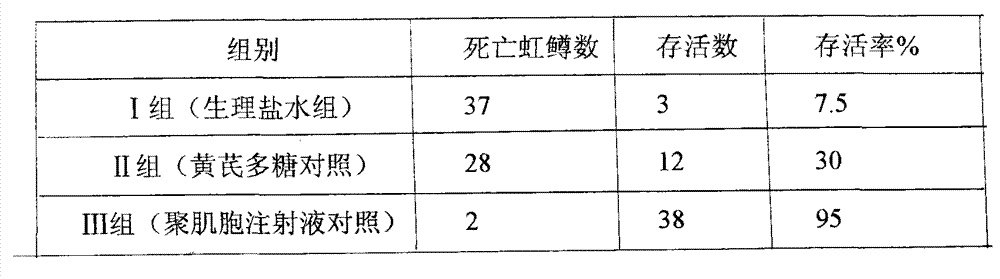
[0022] Embodiment 3: 120 test rainbow trout juveniles were randomly and equally divided into 3 test groups for testing, with 40 fish in each group. Group I: blank control group (normal saline group); Group II: astragalus polysaccharide drug control group (astragalus polysaccharide oral liquid, 30 mg per kilogram of body weight); Group III: polyinosin drug control group (polyinosinic acid solution, 0.05mg per kilogram of body weight). The drugs used in each group were used in the form of medicated baths. The rainbow trout juveniles were isolated and reared in aquariums according to the above experiments, and the feeding conditions were kept the same. After one week of feeding, each group was given medicine according to the plan. During the medicinal bath, most of the water was released from the aquarium, and the medicine was added to maintain oxygenation. The medicinal bath was 30 minutes, and then water was added to a constant value. After 12 hours, add 100 times LD to each ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com