A kind of preparation method of fluorescent polymer with hydrophilic shell and hydrophobic core structure
A fluorescent polymer, core structure technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of unclear degradation mechanism of inorganic quantum dots, decrease in fluorescence intensity, influence detection results, etc. Effects of bleaching and poor stability, increasing fluorescence intensity, and reducing photobleaching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
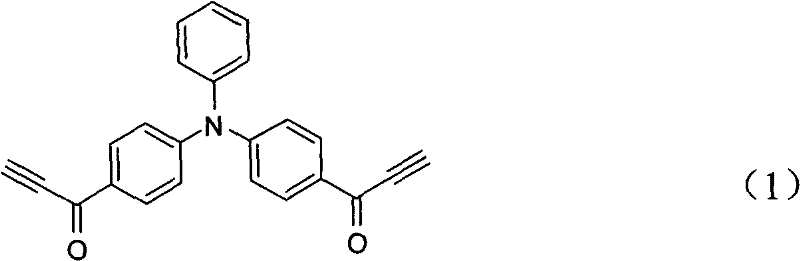
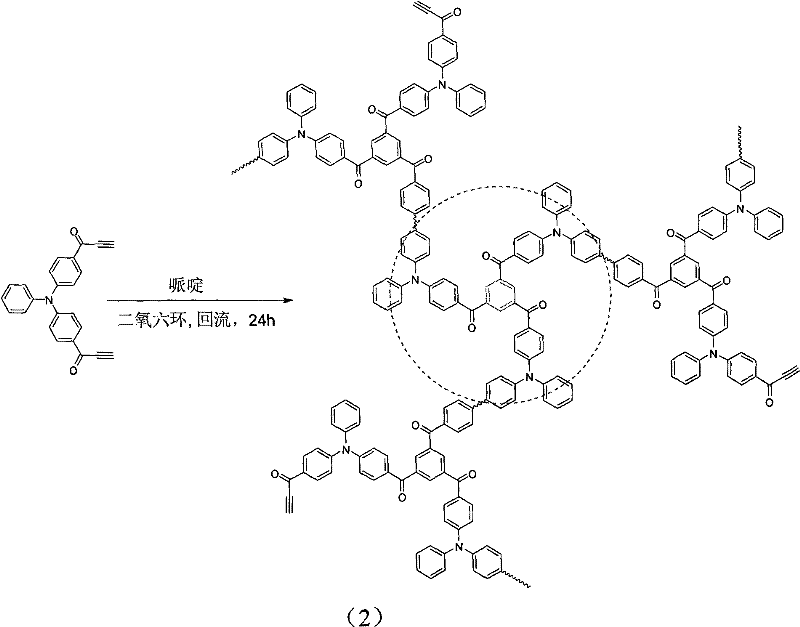
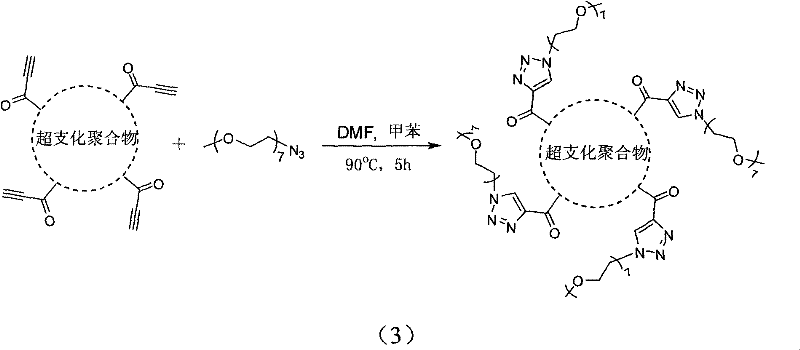
[0021] The invention discloses a method for preparing a fluorescent polymer with a hydrophilic shell and a hydrophobic core structure. The preparation method includes the following steps: (1) A hyperbranched fluorescent polymer is prepared by cyclotrimerization of a diacetylene monomer containing a fluorescent chromophore, and the periphery of the hyperbranched fluorescent polymer has unreacted alkynyl groups; Hydrophilic polymer segments with monofunctional groups can be substituted or coupled to obtain hydrophilic polymer segments that can be added or coupled, or hydrophilic monomers can be polymerized to obtain addition or coupleable hydrophilic Polymer segment; (2) the hyperbranched fluorescent polymer and the hydrophilic polymer segment that can be added or coupled are correspondingly added or coupled to obtain fluorescent polymerization of a hydrophilic shell and a hydrophobic core structure Things.
[0022] The fluorescent polymer obtained by the invention has a core-shel...
Embodiment 1
[0025] Step 1): On the one hand, 36 mL of N,N'-dimethylformamide (DMF) is added to a 100 mL flask. In an ice bath at 0°C, 46 mL of phosphorus oxychloride was added dropwise to the flask with constant stirring. After the addition of phosphorus oxychloride is completed, weigh 4.9 g of triphenylamine and add, remove the ice bath, and raise the temperature to 95°C. After 4 hours of refluxing reaction, extraction treatment, separation and purification by column chromatography, intermediate product 1 was obtained. Add 1.2 g of Intermediate Product 1 to a dry 250 mL two-necked flask to ensure anhydrous and oxygen-free conditions, and add 40 mL of dewatered tetrahydrofuran solution. Under ice bath, add 20 mL 0.5M acetylene magnesium bromide solution in tetrahydrofuran dropwise. The reaction was carried out overnight at room temperature, and the reaction was terminated with saturated aqueous ammonium chloride solution. After the reaction solution was extracted, the organic phase was ...
Embodiment 2
[0035] Step 1): Prepare hb-TPA according to the method of Example 1.
[0036] And the molecular weight 350 in Example 1 polyethylene glycol (PEG 350 -OH) is replaced by polyethylene glycol (PEG 1900 -OH), other preparation conditions are the same as in Example 1, and a polyethylene glycol (PEG) with a molecular weight of 1900 with a single azide end group was prepared 1900 -N 3 ).
[0037] Step 2): Weigh 40mg hb-TPA and 800mg PEG 1900 -N 3 In the polymerization tube, add 1 mL each of DMF and toluene as a solvent, and react at 100°C for 4 hours, so that the hb-TPA has unreacted alkynyl groups and PEG 1900 -N 3 1,3-dipolar cycloaddition reaction between the azido groups. After the reaction is completed, the reaction solution is diluted and precipitated in a large amount of n-hexane, allowed to stand for 24 hours, filtered and dried to constant weight to obtain an amphiphilic hyperbranched fluorescent polymer, that is, a fluorescent polymer with a hydrophilic shell and a hydrophobic co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com